Study on selective separation of vanadium, titanium and tungsten from spent SCR denitration catalyst
-
摘要: 分别采用NaOH、HCl浸出废SCR催化剂,碳酸钠焙烧-水浸废SCR催化剂选择性分离钛。试验表明:碳酸钠焙烧-水浸废催化剂可实现钛与钒、钨高效分离。较优工艺条件:焙烧温度850 ℃,焙烧时间3 h,碳酸钠与废催化剂质量比为1.3,浸出温度95 ℃,浸出时间1 h,搅拌速度500 r/min。V、As、W的浸出率分别为52.26%,98.24%和99.9%。采用硫酸浸出废SCR催化剂钠化焙烧渣实现高效提取钛。工艺条件:上述较优条件焙烧渣,40%硫酸,液固比4∶1,浸出温度90 ℃,浸出时间3 h,搅拌速度500 r/min。钛的浸出率为93.4%。采用自生晶种水解法制备偏钛酸,钛水解率为94.05%,偏钛酸纯度为94.07%。Abstract: Titanium was selectively separated from waste SCR catalyst by NaOH leaching, HCl leaching and sodium carbonate roasting followed by water leaching, respectively. The results show that titanium can be separated from vanadium and tungsten by sodium carbonate roasting and water leaching of the catalyst. The optimum process conditions are as follows: roasting temperature 850 ℃, roasting time 3 h, mass ratio of sodium carbonate to waste catalyst 1.3, leaching temperature 95 ℃, leaching time 1 h, stirring speed 500 r/min. The leaching rates of V, As and W are 52.26%, 98.24% and 99.9%, respectively. High efficient titanium extraction can be achieved by leaching sodium roasting slag of the spent SCR catalyst with sulfuric acid. The optimum conditions are as follows: 40% sulfuric acid, liquid-solid ratio 4∶1, leaching temperature 90 ℃, leaching time 3 h, stirring speed 500 r/min. The leaching rate of titanium is 93.4%. Metatitanic acid was prepared by hydrolysis. The hydrolysis rate of titanium is 94.05% and the purity of metatitanic acid is 94.07%.
-
表 1 废SCR脱硝催化剂化学成分分析
Table 1. Chemical compositions of spent SCR denitration catalyst %
Al As Fe K Mg Si Ti V W 1.03 0.14 0.30 0.12 0.13 4.32 45.12 0.754 2.59 表 2 NaOH浸出废脱硝催化剂浸出液各元素含量
Table 2. Contents of elements in leaching solution of spent denitration catalyst leached by NaOH
NaOH浓度/(g·L−1) 浸出液各元素含量/(mg·L−1) 浸出液体积/mL Al As Mn Mo P S Si Ti V W 50 330.7 148.9 0.25 8.51 10.08 1048 1415 0.41 388.2 838.2 198 100 545.1 176 0.33 11.13 9.81 1220 2409 0.91 463.4 1405 170 200 292.1 207.6 0.46 14.78 11.62 1322 3645 4.78 569.2 2418 160 300 497 184.3 0.55 12.61 8.94 1148 4185 18.93 510.2 2629 188 表 3 盐酸浸出废脱硝催化剂浸出液各元素含量
Table 3. Concentration of elements in solution of spent denitration catalyst leached by HCl solution
盐酸质量分数/% 浸出液中各元素含量/(mg·L−1) 浸出液体积/mL Al As Ca K Mg Na S Si Ti V W 10 124.7 27.57 170.4 167.9 21.93 261 792.3 69.43 115.2 276.3 0.62 230 15 146.5 33.01 183.6 170.2 23.76 232 792 33.45 218.4 290.6 2.43 219 20 114.2 27.8 169 148.9 18.78 203 699.8 18.48 360.2 270.6 5.09 240 表 4 盐酸浸出废脱硝催化剂主要元素浸出率
Table 4. Leaching rate of main elements in waste denitration catalyst leached by hydrochloric acid
盐酸质量分数/% 浸出率/% As V W 10 18.12 33.71 0.02 15 20.65 33.76 0.08 20 19.06 34.45 0.19 表 5 废SCR脱硝催化剂及浸出渣成分分析
Table 5. Chemical compositions of spent SCR denitration catalyst and the leaching residue
% Al As Ca Fe K Mg Si Ti V W 脱硝催化剂 1.03 0.14 1.06 0.30 0.12 0.13 4.32 45.12 0.75 2.59 浸出渣 0.02 0 0.70 0.22 0.021 0.071 0.16 38.88 0.31 0.07 表 6 硫酸浸出Na2CO3焙烧渣浸出液各元素浓度
Table 6. Concentration of elements in leaching solution of Na2CO3 roasting residue leached by sulfuric acid
mg/L Al As Ca Cr Fe K Mg Na Si Ti V W 238.3 0 827.1 3.12 210.9 15.56 156.8 22540 1441 80550 1155 52.75 表 7 硫酸浸出Na2CO3焙烧渣的尾渣成分分析
Table 7. Chemical compositions of tailings from sulfuric acid leaching of Na2CO3 roasting slag
% Al As Ca Fe K Mg Si Ti V W 0.13 0.007 5.53 0.10 0.044 0.025 0.36 48.88 0.42 0.43 表 8 水解前后溶液元素含量
Table 8. Element content of solution before and after hydrolysis
体积/L 溶液中各元素含量/(mg·L−1) Al As Ca Fe K Mg Na Si Ti V W 水解前 0.42 37.12 0.11 463.20 49.79 30.61 62.32 7 346 336.50 9 420.0 115.90 3.21 水解后 1.03 10.29 191.80 12.62 11.30 20.46 3 301 115.90 228.7 2.71 1.15 表 9 自生晶种制备的偏钛酸成分分析
Table 9. Chemical compositions of metatitanic acid prepared by in-situ seed
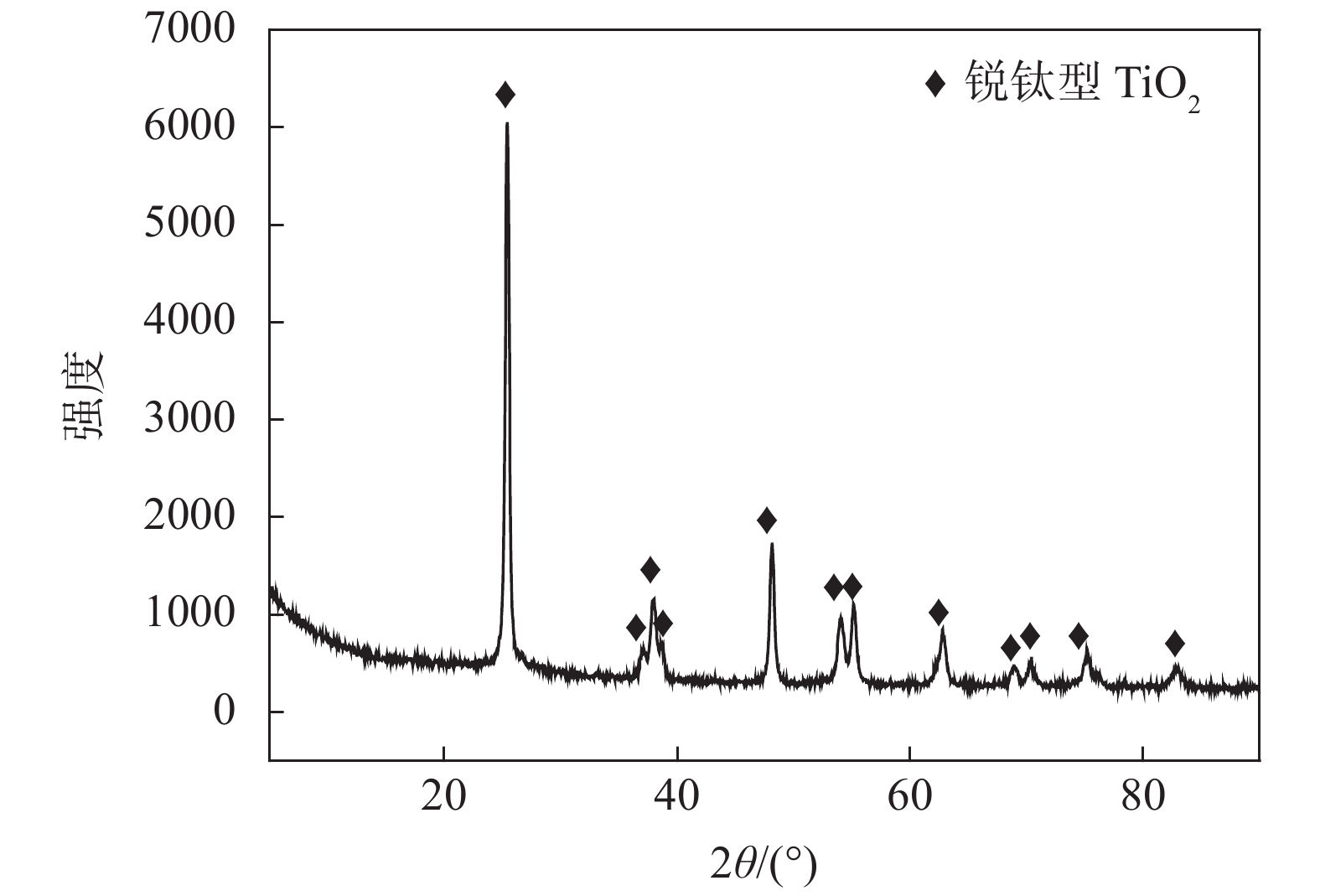
% 类别 Al As Ca Fe K Mg P Si Ti V W 自制偏钛酸 0.005 0.001 0.049 0.080 0.085 0.013 0.003 0.318 46.078 0.384 0.018 工业偏钛酸 0.003 0 0.021 0.085 0.070 0.005 0.067 0.021 49.556 0.473 0.005 -
[1] Zhang Liping, Lv Lingling, Dong Li, et al. Research progress on resources recovery and utilization of waste-SCR-catalyst[J]. Yunnan Chemical Technology, 2019,46(8):77−83. (张立萍, 吕灵灵, 董莉, 等. 废SCR催化剂资源回收利用研究进展[J]. 云南化工, 2019,46(8):77−83. doi: 10.3969/j.issn.1004-275X.2019.08.030 [2] Zeng Rui, Hao Yongli. Analysis on project construction pattern of abandoned SCR catalyzer recovery and utilization[J]. China Environmental Protection Industry, 2014,(9):41−45. (曾瑞, 郝永利. 废弃SCR催化剂回收利用项目建设格局的分析[J]. 中国环保产业, 2014,(9):41−45. doi: 10.3969/j.issn.1006-5377.2014.09.008 [3] LEE Jungbin, EOM Yongseok, KIM Junhan, et al. Regeneration of waste SCR catalyst by air lift loop reactor[J]. Journal of Central South University, 2013,20(5):1314−1318. doi: 10.1007/s11771-013-1617-5 [4] Zeng Rui. Reclamation and recycling of SCR waste catalyzer[J]. China Environmental Protection Industry, 2013,(2):39−42. (曾瑞. 浅谈SCR废催化剂的回收再利用[J]. 中国环保产业, 2013,(2):39−42. doi: 10.3969/j.issn.1006-5377.2013.02.014 [5] Zhang Bingbing, Yu Dandan, Wang Fang, et al. Technology of vanadium recovery from deactivated denitration catalyst[J]. Henan Science, 2016,34(6):866. (张兵兵, 于丹丹, 王芳, 等. 废脱硝催化剂中五氧化二钒回收工艺研究[J]. 河南科学, 2016,34(6):866. [6] Zheng Yilin, Dai Shijin, Zhao Youcai, et al. Selective leaching of vanadium and tungsten from spent SCR catalyst using organic acids[J]. Environmental Protection of Chemical Industry, 2020,40(2):162−168. (郑怡琳, 戴世金, 赵由才, 等. 废SCR催化剂中钒和钨的有机酸浸出[J]. 化工环保, 2020,40(2):162−168. doi: 10.3969/j.issn.1006-1878.2020.02.009 [7] Zeng Xiaoyi, Mei Qizheng, Sun Zhengyuan. High efficient recycling of TiO2 from waste SCR catalyst by Na2CO3 roasting and water leaching[J]. Nonferrous Metals(Extractive Metallurgy), 2019,(12):23−28. (曾小义, 梅其政, 孙正圆. 废SCR催化剂碳酸钠焙烧浸出回收二氧化钛[J]. 有色金属(冶炼部分), 2019,(12):23−28. [8] Liu Zilin, Wang Baodong, Ma Ruixin, et al. Study on mechanism of recovery of tungsten and vanadium from waste SCR catalysts by soda roasting[J]. Inorganic Chemicals Industry, 2016,48(7):63−67. (刘子林, 王宝冬, 马瑞新, 等. 废SCR催化剂钠化焙烧回收钨和钒的机理探究[J]. 无机盐工业, 2016,48(7):63−67. [9] Zhou Kai, Lu Bin, Wang Sheng, et al. Research on recovery process of Ti, V and W in waste SCR denitration catalyst[J]. Electric Power Technology and Environmental Protection, 2019,35(4):8−13. (周凯, 陆斌, 王圣, 等. 废弃SCR脱硝催化剂中Ti、V、W元素回收工艺研究[J]. 电力科技与环保, 2019,35(4):8−13. doi: 10.3969/j.issn.1674-8069.2019.04.003 [10] (李小文. 废SCR催化剂高压碱浸回收钨钒的工艺研究[D]. 赣州: 江西理工大学, 2019.)Li Xiaowen. Recovery of tungsten and vanadium from waste SCR catalyst by high pressure alkaline leaching[D]. Ganzhou: Jiangxi University of Science and Technology, 2019. [11] Chen Yang, Jin Ke, Chen Jiayu, et al. Leaching of V and W from spent SCR catalyst-effect of agitation on leaching rates[J]. Journal of Functional Materials, 2020,51(3):3001−3006. (陈洋, 金科, 陈嘉宇, 等. 废脱硝催化剂钒、钨的浸出-搅拌对浸出率的影响[J]. 功能材料, 2020,51(3):3001−3006. [12] Tang Dingling, Song Hao, Liu Dingding, et al. Study on leaching kinetics of extracting vanadium and tungsten by sodium hydroxide from spent SCR catalyst[J]. Chinese Journal of Environmental Engineering, 2017,11(2):1093−1100. (唐丁玲, 宋浩, 刘丁丁, 等. 废弃脱硝催化剂碱浸提取钒和钨的浸出动力学研究[J]. 环境工程学报, 2017,11(2):1093−1100. doi: 10.12030/j.cjee.201509258 [13] Zhang Chen, Liu Jianhua, Yang Xiaobo, et al. Ultrasound assisted enhancement in vanadium and tungsten leaching from waste SCR catalyst[J]. Functional Materials, 2015,46(20):20063−20067. (张琛, 刘建华, 杨晓博, 等. 超声强化废SCR催化剂浸出V和W的研究[J]. 功能材料, 2015,46(20):20063−20067. doi: 10.3969/j.issn.1001-9731.2015.20.014 [14] Li Wenjun, Xu Tengfei, Liu Xuesong, et al. Effect comparison of microwave heating and muffle heating on treatment of spent SCR catalyst[J]. Environmental Protection of Chemical Industry, 2017,37(5):572−575. (李文军, 许腾飞, 刘雪松, 等. 微波焙烧法与马弗炉焙烧法处理废脱硝催化剂的效果比较[J]. 化工环保, 2017,37(5):572−575. doi: 10.3969/j.issn.1006-1878.2017.05.015 [15] Chen Guangyu, Kang Jialong, Liu Junjie, et al. Study on direct alloying of waste SCR catalysts[J]. Iron Steel Vanadium Titanium, 2018,39(6):99−102. (陈广玉, 康嘉龙, 刘俊杰, 等. 废弃脱硝催化剂直接合金化研究[J]. 钢铁钒钛, 2018,39(6):99−102. doi: 10.7513/j.issn.1004-7638.2018.06.016 [16] Piao Rongxun, Ma Lan, Yang Shaoli, et al. Experimental study on preparation of Cr-containing Ti-Al based alloys by aluminothermic reduction-remelting of waste SCR Ti-based denitration catalyst[J]. Iron Steel Vanadium Titanium, 2019,40(2):79−86. (朴荣勋, 马兰, 杨绍利, 等. 废SCR钛基脱硝催化剂铝热还原重熔制备含铬钛铝基合金的试验研究[J]. 钢铁钒钛, 2019,40(2):79−86. doi: 10.7513/j.issn.1004-7638.2019.02.013 -





 下载:
下载: