Effect of HCl and H2SO4 on SiO2 coating of rutile titanium dioxide
-
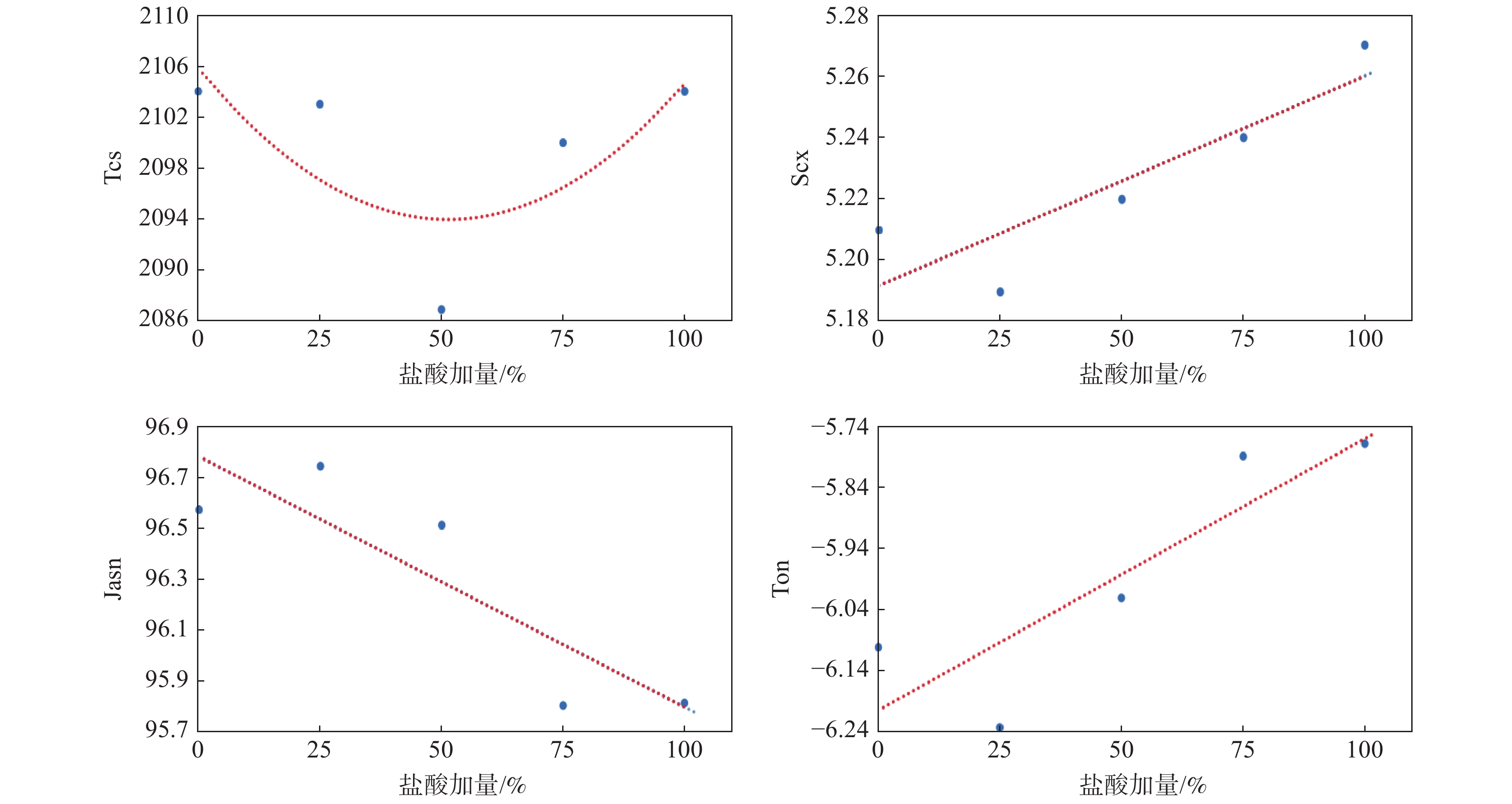
摘要: 选择硅酸钠作为氯化法金红石钛白的包硅原料,首先研究了包膜过程中调节pH的盐酸加量与包膜后钛白粉颜料性能的关系,结果表明随着盐酸加量的增加钛白粉亮度、白度L值和蓝光白度等颜料性能指标降低,但Scx和b值等蓝相指标则逐渐改善。其次对比分析了包硅过程中硫酸和盐酸两种不同酸体系中钛白粉二氧化硅膜层均匀致密性、颜料性能和应用性能的差距,明确了硫酸体系的二氧化硅膜层均匀致密性更好、光泽度更高,而盐酸体系的蓝相更优。Abstract: Using sodium silicate as the coating agent for rutile titanium dioxide from chlorination process, the relationship between the pigmentary properties of the coated titanium dioxide and the addition amount of hydrochloric acid used for pH adjustment during the coating process, was investigated firstly. The results show that with increase of the hydrochloric acid addition, the brightness, whiteness L value and blue whiteness of the titanium dioxide decrease, while the blue phase indexes (e.g. Scx and b value) of the titanium dioxide are improved gradually. Subsequently, the homogeneity and compactness of the silicon dioxide coating layer, pigmentary and application properties of the titanium dioxide from sulfuric acid and hydrochloric acid systems were compared and analyzed. A better homogeneity and compactness coupled with a higher glossiness of the silicon dioxide coating layer can be obtained for titanium dioxide from sulfuric acid system, while the titanium dioxide from hydrochloric acid system has a better blue phase.
-
Key words:
- rutile TiO2 /
- inorganic coating /
- HCl /
- H2SO4 /
- pigmentary properties
-
表 1 仪器设备
Table 1. Instruments and equipment
名称 型号 厂家 平磨仪 PM240-2 广西梧州市润讯宝石机械厂 台式色彩检测仪 800 V Datacolor 光泽仪 GM-268Plus Konica Minolta 场发射透射电子
显微镜Talos F200S Thermo Fisher Scientific
(原FEI)表 2 HCl和H2SO4体系的包膜样颜料性能对比
Table 2. Pigment characteristics of inorganic-processed titanium dioxide from HCl and H2SO4 systems
编号 脱氯 体系 Jasn Ton L a b R457 1 是 硫酸 96.16 −5.86 96.61 −1.04 −0.06 92.18 2 否 硫酸 96.48 −5.83 96.91 −0.97 0.13 92.67 3 否 盐酸 95.41 −5.32 96.11 −1.10 −0.25 91.21 表 3 HCl和H2SO4体系的包膜样光泽度对比
Table 3. Glossiness performance of inorganic-processed titanium dioxide from HCl and H2SO4 systems
编号 脱氯 体系 光泽度 20° 60° 85° 1 是 硫酸 37.4 82.6 93.5 2 否 硫酸 38.5 83.2 94.4 3 否 盐酸 36.2 82.9 93.3 -
[1] Tracy L T. Photocatalysis on titanium dioxide surfaces[D]. USA: University of Pittsburgh, 2006: 3-33. [2] Ghiaci M, Aghaei H, Abbaspur A. Size-controlled synthesis of ZrO2–TiO2 nanoparticles prepared via reverse micelle method: Investigation of particle size effect on the catalytic performance in vapor phase beckmann rearrangement[J]. Materials Research Bulletin, 2008,43(5):1255−1262. doi: 10.1016/j.materresbull.2007.05.022 [3] Palanisamy B, Babu C M, Sundaravel B, et al. Sol–gel synthesis of mesoporous mixed Fe2O3/TiO2 photocatalyst: Application for degradation of 4-chlorophenol[J]. Journal of Hazardous Materials, 2013,252-253:233−242. doi: 10.1016/j.jhazmat.2013.02.060 [4] Pekka I. Coated titanium dioxide pigment and a process for the production of the same, USA: US RE31602 E[P]. 1984. [5] Sami M B. Methof for surface treatment of titanium dioxide pigment, USA: US20090087556[P]. 2015. [6] Comsup N, Panpranot J, Praserthdam P. The influence of Si-modified TiO2 on the activity of Ag/TiO2 in CO oxidation[J]. Journal of Industrial and Engineering Chemistry, 2010,16(5):703−707. doi: 10.1016/j.jiec.2010.07.015 [7] Kim J Y, Kim C S, Chang H K, et al. Effects of ZrO2 addition on phase stability and photocatalytic activity of ZrO2/TiO2 nanoparticles[J]. Advanced Powder Technology, 2010,21(2):141−144. doi: 10.1016/j.apt.2009.12.008 [8] Zhang Y, Yin H, Wang A, et al. Deposition and characterization of binary Al2O3/SiO2 coating layers on the surfaces of rutile TiO2 and the pigmentary properties[J]. Applied Surface Science, 2010,257(4):1351−1360. doi: 10.1016/j.apsusc.2010.08.071 [9] Liu Y, Ge C, Ren M, et al. Effects of coating parameters on the morphology of SiO2-coated TiO2 and the pigmentary properties[J]. Applied Surfacence, 2008,254(9):2809−2819. doi: 10.1016/j.apsusc.2007.10.021 -





 下载:
下载: