Mathematical modeling and analysis of vanadium extraction from acid leaching solution of clay vanadium ore with N235
-
摘要: 以N235从黏土钒矿酸浸液中提钒的条件试验为基础,主要研究溶剂萃取过程中影响萃取率的因素,确定了pH、萃取剂浓度、相比对萃取率的影响,而后运用响应曲面法确立了三个因素之间的交互效应,并且运用热力学、溶液化学分析了交互作用产生的原因,指出萃取体系中pH与硫元素会极大地影响溶液中的含钒组分,进而影响萃取率,同样含钒组分的变化也会影响萃取效率。总之,萃取体系产生交互作用的核心机理为pH变化引起溶液组分的变化以及萃取剂面对不同离子时所表现的差异性。Abstract: Based on the experiments of extracting vanadium from acid leaching solution of clay vanadium ore with N235, the factors influencing the extraction rate were studied. The influences of pH value, extractant concentration and phase ratio on the extraction rate were determined. The interaction effect during the three factors was determined by response surface methodology. The causes of interaction were analyzed by thermodynamics and solution chemistry. It is shown that the pH value and sulfur content in the extraction system greatly affect the vanadium components in the solution, and then affect the extraction rate. Meanwhile, the change of vanadium components also affects the extraction rate. Conclusively, the core mechanism of the interaction of the extraction system is the change of solution compositions caused by pH variation and the difference of extractant when facing different ions.
-
表 1 浸出液化学成分分析
Table 1. Chemical compositions of leachate
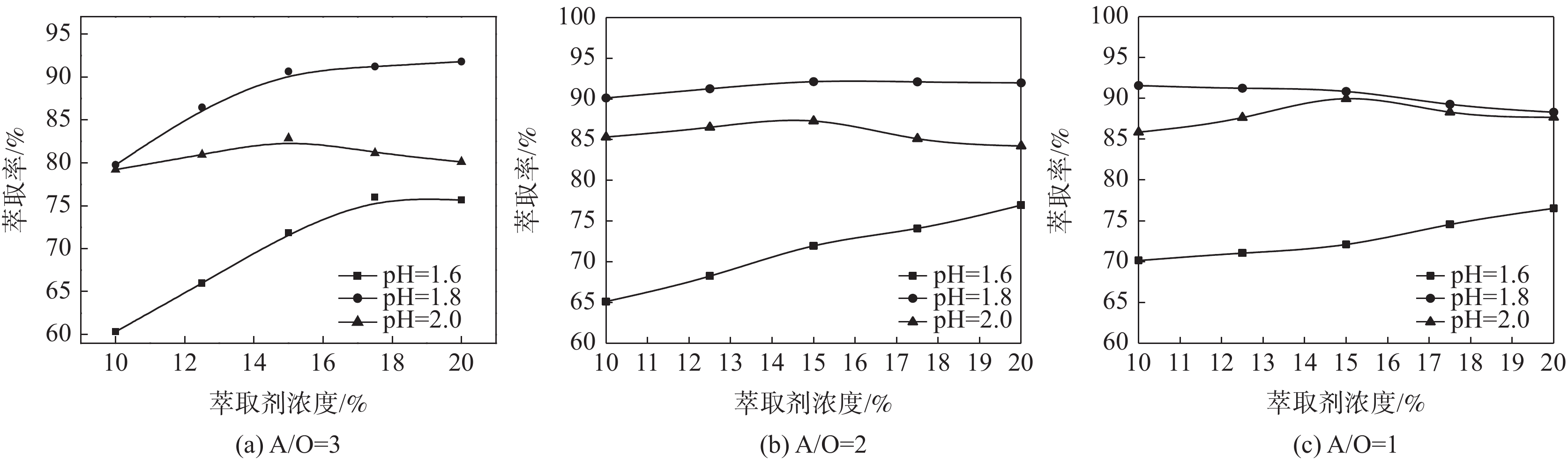
g/L V2O5 Fe Al2O3 SiO2 MgO CaO Na Mn P Cr As 9.38 6.56 16.57 <0.01 2.03 0.096 0.044 3.97 0.97 0.42 <0.1 表 2 条件优化Box-Behnken试验因素与水平
Table 2. Condition optimization Box-Behnken test factors and levels
水平 (A)萃取剂浓度/% (B)pH (C)相比(A/O) −1 10 1.6 3 0 15 1.8 2 1 20 2.0 1 表 3 三因素条件优化Box-Behnken试验因素与水平
Table 3. Three-factor condition optimization Box-Behnken test factors and levels
编号 因素 萃取率/% 萃取剂浓度/% pH 相比(A/O) 1 10 1.6 2.0 65.12 2 20 1.6 2.0 76.95 3 10 2.0 2.0 85.32 4 20 2.0 2.0 84.22 5 10 1.8 1.0 91.58 6 20 1.8 1.0 88.31 7 10 1.8 3.0 79.75 8 20 1.8 3.0 91.8 9 15 1.6 1.0 72.1 10 15 2.0 1.0 89.97 11 15 1.6 3.0 71.87 12 15 2.0 3.0 82.85 13 15 1.8 2.0 91.85 14 15 1.8 2.0 91.9 15 15 1.8 2.0 92.35 16 15 1.8 2.0 92.03 17 15 1.8 2.0 91.01 表 4 响应面试验结果方差分析
Table 4. Analysis of variance of response surface test results
方差来源 平方和 自由度 均方差 F 值 P值 显著性 模型 1187.05 9 131.89 506.19 < 0.0001 ** A 47.58 1 47.58 182.6 < 0.0001 ** B 396.49 1 396.49 1521.67 < 0.0001 ** C 30.77 1 30.77 118.1 < 0.0001 ** AB 41.8 1 41.8 160.41 < 0.0001 ** AC 58.68 1 58.68 225.19 < 0.0001 ** BC 11.87 1 11.87 45.55 0.0003 ** A2 29.16 1 29.16 111.9 < 0.0001 ** B2 537.07 1 537.07 2061.18 < 0.0001 ** C2 7.52 1 7.52 28.86 0.001 ** 残差 1.82 7 0.26 失拟性 0.84 3 0.28 1.13 0.4377 纯误差 0.99 4 0.25 总差 1188.88 16 R2Adj S/N= 注:“*”表示对结果影响显著(P<0.05);“**”表示对结果影响极显著(P<0.01)。 表 5 各因素影响机理分析
Table 5. Analysis of the influence mechanism of various factors
编号 方程式 $ \mathrm{l}\mathrm{g}{K}^{0} $ (1) $ {\text{VO}}_{4}^{3-} $+4H+=$ {\text{VO}}_{\text{2}}^{\text{+}} $+2H2O 24.7 (2) $ {\text{VO}}_{4}^{3-} $+H+=${\rm{HVO}}_4^{2 - } $ 11.54 (3) $ {\text{VO}}_{4}^{3-} $+2H+=${{\rm{H}}_2}{\rm{VO}}_4^ - $ 21.1 (4) 2$ {\text{VO}}_{4}^{3-} $+5H+=$ {{\rm{H}}_3}{{\rm{V}}_2}{\rm{O}}_7^ - $ 46.45 (5) $ {\text{VO}}_{4}^{3-} $+4H++$ \mathrm{S}{\mathrm{O}}_{4}^{2-} $=VO2$ \mathrm{S}{\mathrm{O}}_{4}^{-} $+2H2O 29.37 (6) VO++H+=V(OH)2+ −1.83 (7) V(OH)2++H+=H2O+V3+ 2.96 (8) 4$ {\text{VO}}_{4}^{3-} $+8H+=V4O124− 95.11 (9) 10$ {\text{VO}}_{4}^{3-} $+25H+=HV10O285−+12H2O 270.86 (10) 10$ {\text{VO}}_{4}^{3-} $+26H+=HV10O284−+12H2O 274.49 (11) Fe3++OH−=Fe(OH)3+ 11.89 (12) Fe3++2OH−=$ \text{Fe(OH}{\text{)}}_{2}^{\text{+}} $ 23.62 (13) Fe3++2OH−=$ \text{Fe(OH}{\text{)}}_{2}^{\text{+}} $ 35.3 (14) Fe3++$ \mathrm{S}{\mathrm{O}}_{4}^{2-} $=$ \mathrm{F}\mathrm{e}\text{S}{\text{O}}_{4}^{+} $ 2.03 (15) Fe3++2$ \mathrm{S}{\mathrm{O}}_{4}^{2-} $=$ \mathrm{F}\mathrm{e}(\text{S}{\text{O}}_{\text{4}}{)}_{2}^{-} $ 2.98 (16) H+$ \mathrm{H}\mathrm{S}{\mathrm{O}}_{4}^{-} $=H2SO4 −11.6 (17) H+$ \mathrm{S}{\mathrm{O}}_{4}^{2-} $=$ \mathrm{H}\mathrm{S}{\mathrm{O}}_{4}^{-} $ 1.96 (18) H++OH−=H2O 14 -
[1] Ye Guohua, Hu Yibo, Tong Xiong, et al. Extraction of vanadium from direct acid leaching solution of clay vanadium ore using solvent extraction with N235[J]. Hydrometallurgy, 2018,177:27−33. doi: 10.1016/j.hydromet.2018.02.004 [2] Wen Jiawei, Ning Pengge, Cao Hongbin, et al. Modeling of liquid-liquid extraction of vanadium with primary amine N1923 in H2SO4 medium[J]. Hydrometallurgy, 2018,177:57−65. doi: 10.1016/j.hydromet.2018.02.013 [3] Jing Xiaohua, Ning Pengge, Cao Hongbin, et al. Separation of V(Ⅴ) and Cr(Ⅵ) in leaching solution using annular centrifugal contactors[J]. Chemical Engineering Journal, 2017,315:373−381. doi: 10.1016/j.cej.2017.01.014 [4] Hu J S, Zou D, Chen J, et al. A novel synergistic extraction system for the recovery of scandium (III) by Cyanex272 and Cyanex923 in sulfuric acid medium[J]. Separation and Purification Technology, 2020,233(15):59−77. [5] Kuang S T, Zhang Z F, Li Y L, et al. Synergistic extraction and separation of rare earths from chloride medium by the mixture of HEHAPP and D2EHPA[J]. Hydrometallurgy, 2017,174:78−83. doi: 10.1016/j.hydromet.2017.09.011 [6] Zhang Y, Zhang T G, Lü G Z, et al. Synergistic extraction of vanadium(IV) in sulfuric acid media using a mixture of D2EHPA and EHEHPA[J]. Hydrometallurgy, 2016,166:87−93. doi: 10.1016/j.hydromet.2016.09.003 [7] Zhang Ting'an, Mou Wangzhong, Dou Zhihe, et al. Potential—pH diagrams for V-Fe-H2O system during oxygen pressure acid leaching of vanadium-bearing converter slags[J]. Chinese Journal of Nonferrous Metals, 2011,21(11):2936−2945. (张廷安, 牟望重, 豆志河, 等. 转炉钒渣氧压酸浸过程V-Fe-H2O系的电位-pH图[J]. 中国有色金属学报, 2011,21(11):2936−2945. [8] (杨显万. 高温水溶液热力学数据计算手册[M]. 北京: 冶金工业出版社, 1983: 523-674.)Yang Xianwan. Handbook of thermodynamic data in aqueous solutions at high temperature[M]. Beijing: Metallurgical Industry Press, 1983: 523-674. [9] (伊赫桑·巴伦. 纯物质热化学数据手册[M]. 程乃良, 牛四通, 徐桂英, 译. 北京: 科学出版社, 2003: 716-726.)Barin I. Thermochemical data of pure substances[M]. Cheng Nailiang, Niu Sitong, Xu Guiying, transl. Beijing: Science Press, 2003: 716-726. [10] Chen B F, Huang S, Liu B, et al. Thermodynamic analysis for separation of vanadium and chromium in V(IV)-Cr(III)-H2O system[J]. Transactions of Nonferrous Metals Society of China, 2018,28(3):196. [11] Liu Jingwen, Yang Zhengfei, Zhou Peng, et al. V(Ⅴ)-Fe(Ⅲ)-S(Ⅵ)-H2O thermodynamic study and separation theory of ferrovanadium[J]. Chinese Journal of Nonferrous Metals, 2020,30(4):912−919. (刘景文, 阳征斐, 周鹏, 等. V(Ⅴ)-Fe(Ⅲ)-S(Ⅵ)-H2O系热力学研究与钒铁分离方法理论[J]. 中国有色金属学报, 2020,30(4):912−919. doi: 10.11817/j.ysxb.1004.0609.2020-35755 -





 下载:
下载: