Solid phase reaction and diffusion behavior of V2O5/Cr2O3-CaO system based on calcification roasting of chromium–containing vanadium slag
-
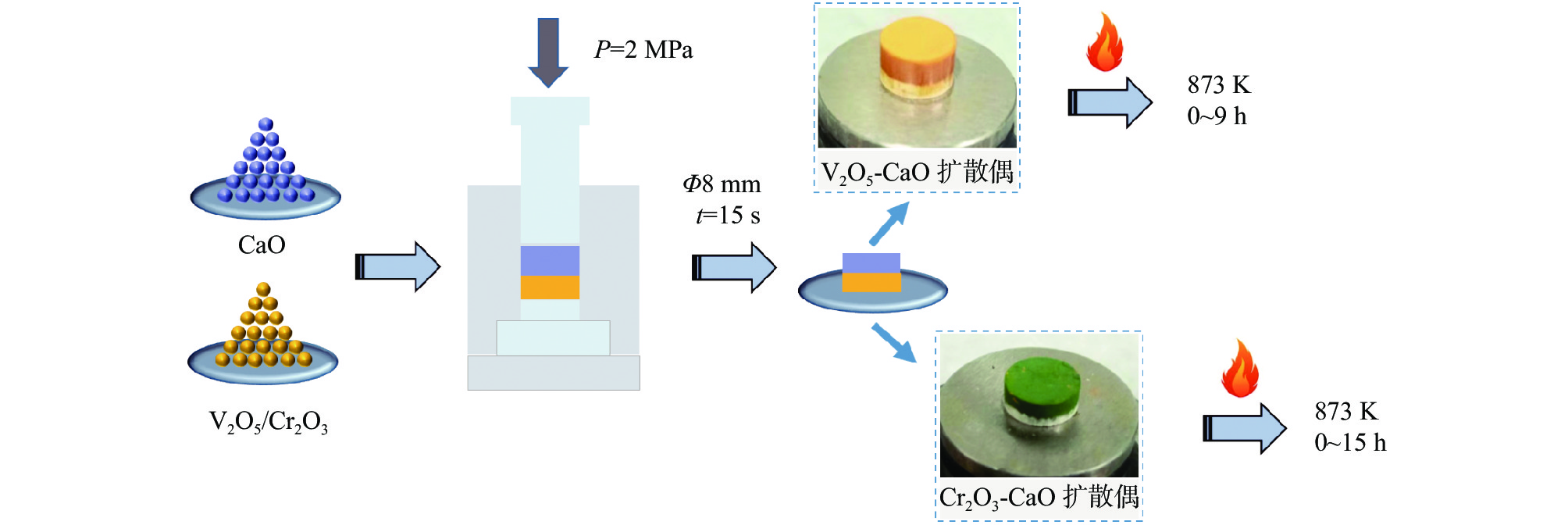
摘要: 为探究钒铬渣钙化焙烧过程钒、铬组元与钙反应能力的差异,在热力学分析基础上,以V2O5、Cr2O3、CaO纯物质为原料,采用恒温焙烧法分别制备出V2O5-CaO与Cr2O3-CaO扩散偶。利用扫描电镜与能谱仪观测不同恒温时间后扩散界面的微观形貌以及产物层元素分布,分析恒温时间对固相界面反应的影响,并利用wagner方程对体系互扩散系数进行计算。结果表明:在空气气氛中873 K恒温焙烧9 h后,V2O5-CaO扩散偶界面产物层清晰,厚度与时间的平方根存在线性关系,说明该固相反应由扩散控速,产物以CaV2O6为主。而相同焙烧条件下,Cr2O3-CaO间未见明显的界面产物层,说明V2O5-CaO间固-固界面反应能力更强,其互扩散系数量级为10−10 cm2·s−1。Abstract: The difference in combining ability with calcium between vanadium and chromium in the calcification roasting process of chromium-containing vanadium slag was investigated by V2O5-CaO and Cr2O3-CaO diffusion couples which were prepared by roasting at constant temperature with V2O5, Cr2O3 and CaO as raw materials. The microstructure of the diffusion interface and the element distribution of the product layer were observed and detected by SEM-EDS. The effect of roasting time on solid phase interface reaction were analyzed, and the diffusion coefficient was calculated by Wagner equation. The results show that a recognizable product layer at the interface of the V2O5-CaO diffusion couple can be found by roasting at 873 K for 9 h in air atmosphere. The thickness of the product layer is linearly related to the square root of roasting time, indicating a diffusion-controlled process for the solid phase reaction, with the product layer mainly composed of CaV2O6. Under the same roasting condition, no obvious product layer is formed in Cr2O3-CaO diffusion couple. It suggests that the solid-solid interface reaction ability between vanadium and calcium is stronger than that of chromium and calcium. The order of magnitude of diffusion coefficient of V2O5-CaO diffusion couple is 10−10 cm2·s−1.
-
表 1 扩散试验条件
Table 1. Conditions of diffusion experiment
扩散偶 温度/K 气氛 恒温扩散时间/h V2O5-CaO 873 空气 0,3,6,9 Cr2O3-CaO 873 空气 0,3,6,9,12,15 表 2 873 K恒温焙烧9 h的V2O5-CaO扩散偶互扩散系数
Table 2. Diffusion coefficient of V2O5-CaO diffusion couple at 873 K for 9 h
恒温时间/
hD×1010(NCa=0.02±0.002)/
(cm2∙s−1)D×1010(NCa=0.05±0.002)/
(cm2∙s−1)D×1010(NCa=0.12±0.01)/
(cm2∙s−1)D×1010(NCa=0.48±0.01)/
(cm2∙s−1)9 0.196 0.141 1.137 0.017 -
[1] Chu Mansheng, Tang Jue, Liu Zhenggen, et al. The extraction of vanadium from fired titanovanadium-bearing magnetite pellets with sodium sulphate addition[J]. Journal of Iron and Steel Research, 2017,29(5):335−344. (储满生, 唐珏, 柳政根, 等. 高铬型钒钛磁铁矿综合利用现状及进展[J]. 钢铁研究学报, 2017,29(5):335−344. [2] Tang Jue, Zhang Yong, Chu Mansheng, et al. Preparation of oxidized pellets with high chromium vanadium-titanium magnetite[J]. Journal of Northeastern University (Natural Science), 2013,34(4):545−550. (唐珏, 张勇, 储满生, 等. 以高铬型钒钛磁铁矿制备氧化球团[J]. 东北大学学报(自然科学版), 2013,34(4):545−550. doi: 10.3969/j.issn.1005-3026.2013.04.022 [3] Gao Shimin. Discussion on smelting technology of a high chromium vanadium titanium magnetite[J]. Iron Steel vanadium Titanium, 2020,41(5):27−36. (高师敏. 一种高铬型钒钛磁铁矿冶炼工艺探讨[J]. 钢铁钒钛, 2020,41(5):27−36. [4] (高官金. 高铬钒渣的综合利用[D]. 北京: 中国科学院大学, 2017.)Gao Guanjin. Study on comprehensive utilization of high-chromium vanadium slag[D]. Beijing: Unversity of Chinese Academy of Sciences, 2017. [5] Jiang Tao, Wen Jing, Zhou Mi, et al. Phase evolutions, microstructure and reaction mechanism during calcification roasting of high chromium vanadium slag[J]. Journal of Alloys & Compounds, 2018,742:402−412. [6] Wen Jing, Jiang Tao, Xv Yingzhe, et al. Efficient separation and extraction of vanadium and chromium in high chromium vanadium slag by selective two-stage roasting-leaching[J]. Metallurgical and Materials Transactions B, 2018,49(3):1471−1481. doi: 10.1007/s11663-018-1197-8 [7] Wang Mingyu, Zhou Shengfan, Wang Xuewenw, et al. Recovery of iron from chromium vanadium-bearing titanomagnetite concentrate by direct reduction[J]. Journal of the Minerals Metals & Materials Society, 2016,68(10):2698−2703. [8] (李晓军. 钒渣中尖晶石生长规律及钒渣钙化焙烧机理的研究[D]. 重庆: 重庆大学, 2011.)Li Xiaojun. Research on spinels growth law and calcification roasting mechanism of vanadium slag[D]. Chongqing: Chongqing Unversity, 2011. [9] Yang Yang, Mao Huahai, Selleby Malin, et al. An assessment of the Ca-V-O system[J]. Calphad: Computer Coupling of Phase Diagrams and Thermochemistry, 2017,56:29−40. doi: 10.1016/j.calphad.2016.11.005 [10] Wang Zhenghao, Chen Liang, Aldahrib Tahani, et al. Direct recovery of low valence vanadium from vanadium slag―Effect of roasting on vanadium leaching[J]. Hydrometallurgy, 2020:191. [11] Wen Jing, Jiang Tao, Wang Junpeng, et al. An efficient utilization of high chromium vanadium slag: Extraction of vanadium based on manganese carbonate roasting and detoxification processing of chromium-containing tailings[J]. Journal of Hazardous Materials, 2019:378. [12] Cao Peng. Research on vanadium slag roasted with calcium salt[J]. Iron Steel vanadium Titanium, 2012,33(1):30−34. (曹鹏. 钒渣钒渣钙化焙烧试验研究[J]. 钢铁钒钛, 2012,33(1):30−34. doi: 10.7513/j.issn.1004-7638.2012.01.006 [13] Zhang Juhua, Zhang Wei, Yang Yongxia, et al. Influencing factors of vanadium slag roasting with calcium and oxidation kinetics for roasting process[J]. Journal of Northeastern University (Natural Science), 2014,35(6):831−835. (张菊花, 张伟, 杨勇霞, 等. 钒渣钙化焙烧的影响因素及焙烧氧化动力学[J]. 东北大学学报(自然科学版), 2014,35(6):831−835. doi: 10.3969/j.issn.1005-3026.2014.06.016 [14] Fu Nianxin, Zhang Lin, Liu Wuhan, et al. Behavior of phase transformations in oxidation of vanadium slags with participation of limestone[J]. The Chinese Journal of Process Engineering, 2017,17(2):285−291. (付念新, 张林, 刘武汉, 等. 钒渣添加石灰石氧化焙烧过程物相转化行为[J]. 过程工程学报, 2017,17(2):285−291. doi: 10.12034/j.issn.1009-606X.216297 [15] Akira Shimizu, Saitou Junji, Hao Yujun, et al. Effect of contact points between particles on the reaction rate in the Fe2O3-V2O5 system[J]. Solid State Lonics, 1990,38(3−4):261−269. doi: 10.1016/0167-2738(90)90431-P [16] Chen Bojian, Zhou Mi, Jiang Tao, et al. Observation of diffusion behavior between Cr2O3 and calcium ferrite based on diffusion couple method for 1373K[J]. Journal of Alloys & Compounds, 2019,802:103−111. [17] Wu Dousi, Lu Shuqin, Lu Jinsuo. The hydrogenoid calculation formula of cation radius[J]. Chinese Science Bulletion, 1987,(3):195−199. (吴斗思, 卢淑琴, 卢锦梭. 阳离子半径的类氢化计算公式[J]. 科学通报, 1987,(3):195−199. [18] Ren Zhongshan, Hu Xiaojun, Xue Xiangxin, et al. Solid state reaction studies in Fe3O4-TiO2 system by diffusion couple method[J]. Journal of Alloys & Compounds, 2013,580:182−186. [19] Jin Mingfang, Li Guangsen, Chu Mansheng, et al. Diffusion between MgO and hematite during sintering[J]. Iron and Steel, 2008,43(3):10−14. (金明芳, 李光森, 储满生, 等. 烧结过程中MgO在赤铁矿中的扩散行为的研究[J]. 钢铁, 2008,43(3):10−14. doi: 10.3321/j.issn:0449-749X.2008.03.002 [20] Wagner C. The evaluation of data obtained with diffusion couples of binary single-phase and multiphase systems[J]. Acta Metallurgica, 1969,17(2):99−107. doi: 10.1016/0001-6160(69)90131-X -





 下载:
下载: