Synthesis of anatase TiO2 nanorods with special exposed surface in a novel solvothermal system
-
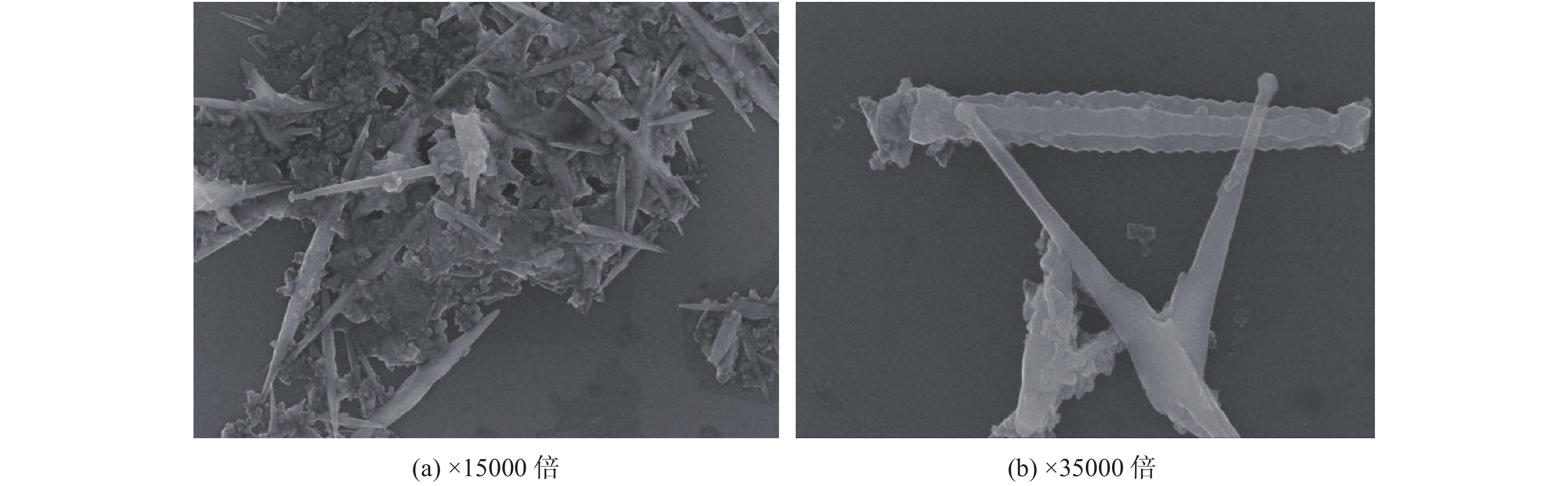
摘要: 以四丁基氢氧化铵(TBAH)为形貌控制剂,采用一种新的无氟溶剂热反应体系,实现了特定晶面的可控合成,制备出了锐钛矿型TiO2单晶纳米棒材料。所获得的TiO2纳米棒主要由表面的{010}小平面控制。用该纳米棒制成的染料敏化电池(DSSCs)的短路电流密度Jsc约为10.9 mA/cm2,开路电压Voc约为0.74 V,光电功率转换效率约为5.75%;比用商业P25型 TiO2制成的DSSCs具有更为优异的电池性能,电池的短路电流密度、填充因子、功率转换效率分别提高了2.83%、10.94%和10.58%。在材料表征的基础上,对其形成机理进行了初步的探讨。
-
关键词:
- 锐钛矿TiO2纳米棒 /
- 晶面 /
- 燃料敏化电池 /
- 功率转换效率
Abstract: Through a novel solvothermal method, single-crystalline anatase TiO2 nanorods were prepared using tetrabutylammonium hydroxide (TBAH) as the morphology controlling agent. The obtained TiO2 nanorods are dominated by a large percentage of {010} facets on the surface. The short-circuit current density Jsc of the dye-sensitized solar cells (DSSCs) made of the TiO2 nanorods is about 10.9 mA/cm2, with the open circuit voltage Voc and photoelectric conversion efficiency at 0.74 V and 5.75%, respectively. Compared with DSSCs made of commercial P25 TiO2, the short-circuit current density, fill factor, and photoelectric conversion efficiency of the cell made of the TiO2 nanorods are increased by 2.83%, 10.94% and 10.58%, respectively. On basis of the material characterizations, the formation mechanisms were discussed preliminarily.-
Key words:
- anatase TiO2 nanorods /
- facet /
- dye-sensitized solar cell /
- power conversion efficiency
-
图 5 TiO6 八面体链接聚合物示意[18]
Figure 5. Schematic diagram of TiO2 octahedral linked polymer
表 1 由P25 TiO2和TiO2纳米棒制成的DSSC的电池参数
Table 1. DSSC parameters of battery respectively made of P25 TiO2 and TiO2 nanorods
样品 Voc/V Jsc/(mA· cm−2) FF η/% P25 TiO2 0.77 10.6 0.64 5.20 TiO2纳米棒 0.74 10.9 0.71 5.75 -
[1] Kavan L, Gratzel M, Gilbert S E, et al. Electrochemical and photoelectrochemical investigation of single-crystal anatase[J]. J. Am. Chem. Soc., 1996,118:6716−6723. doi: 10.1021/ja954172l [2] Chen J S, Tan Y L, Li C M, et al. Constructing hierarchical spheres from large ultrathin anatase TiO2 nanosheets with nearly 100% exposed (001) facets for fast reversible lithium storage[J]. J. Am. Chem. Soc., 2010, 132: 6124−6130. https://pubmed.ncbi.nlm.nih.gov/20392065. [3] Linsebigler A L, Lu G Q, Yates J T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results[J]. Chem. Rev., 1995, 95: 735−758. https://www.scirp.org/reference/ReferencesPapers.aspx?ReferenceID=2113324. [4] Mao Y, Wong S S. Size- and shape-dependent transformation of nanosized titanate into analogous anatase titania nanostructures[J]. J. Am. Chem. Soc., 2006,128:8217−8226. doi: 10.1021/ja0607483 [5] Amano F, Yasumoto T, Prieto-Mahaney, et al. Photocatalytic activity of octahedral single-crystalline mesoparticles of anatase titanium(IV) oxide[J]. Chem. Commun., 2009, 17: 2311–2313. https://pubs.rsc.org/en/content/articlelanding/2009/cc/b822634b#!divAbstract. [6] Zhang Z, Wang C C, Zakaria R, et al. Role of particle size in nanocrystalline TiO2-based photocatalysts[J]. J. Phys. Chem. B, 1998,102:10871−10878. doi: 10.1021/jp982948%2B [7] YangW G, Wan F R, Chen Q W, et al. Controlling synthesis of well-crystallized mesoporous TiO2 microspheres with ultrahigh surface area for high-performance dye-sensitized solar cells[J]. J. Mater. Chem., 2010, 20: 2870−2876. https://pubs.rsc.org/en/content/articlelanding/2010/JM/B923105F#!divAbstract. [8] Yang W, Li J, Wang Y, et al. A facile synthesis of anatase TiO2 nanosheets-based hierarchical spheres with over 90% {001} facets for dye-sensitized solar cells[J]. Chem. Commun., 2011, 47: 1809−1811. https://pubs.rsc.org/en/content/articlelanding/2011/CC/C0CC03312J#!divAbstract. [9] Ferber J, Luther J. Computer simulations of light scattering and absorption in dye-sensitized solar cells[J]. Sol. Energy Mater. Sol. Cells, 1998,54:265−275. doi: 10.1016/S0927-0248(98)00078-6 [10] TachibanaY, Sayama K, Arakawa H. Quantitative analysis of light-harvesting efficiency and electron-transfer yield in ruthenium-dye-sensitized nanocrystalline TiO2 solar cells[J]. Chem. Mater., 2002, 14: 2527−2535. https://pubs.acs.org/toc/cmatex/14/6. [11] Liu Leng, En Yi. Synthesis, transformation mechanism and photocatalytic properties of various morphologies anatase TiO2 nanocrystals derived from tetratitanate nanobelts[J]. Chemistry Select, 2018,3:9953 −9959. [12] Pu M. Anisotropic meta-mirror for achromatic electromagnetic polarization manipulation[J]. Appl. Phys. Lett., 2013,102:131906. doi: 10.1063/1.4799162 [13] Lazzeri M, Vittadini A, Selloni A. Erratum: Structure and energetics of stoichiometric TiO2 anatase surfaces[J]. Phys. Rev. B, 2002,65(11):119901. doi: 10.1103/PhysRevB.65.119901 [14] Wen P, Ishikawa Y, Itoh H, et al. Topotactic transformation reaction from layered titanate nanosheets into anatase nanocrystals[J]. J Phys Chem C, 2009,113:20275−20280. doi: 10.1021/jp908181e [15] Wu B, Guo C, Zheng N, et al. Nonaqueous production of nanostructured anatase with high-energy facets[J]. J. Am. Chem. Soc., 2008,130:17563−17567. doi: 10.1021/ja8069715 [16] Liu Jin, Luo Jun, Yang Weiguang, et al. Synthesis of single-crystalline anatase TiO2 nanorods with high-performance dye-sensitized solar cells[J]. J. Mater. Sci. Technol., 2015,31(1):106−109. doi: 10.1016/j.jmst.2014.07.015 [17] Liu C M, Yang S H. Synthesis of angstrom-scale anatasetitania atomic wires[J]. Acs Nano, 2009,3(4):1025−1031. doi: 10.1021/nn900157r [18] Chemseddine A, Moritz T. Nanostructuringtitania control over nanocrystals structure, size, shape, and organization[J]. Eur. J. Inorg. Chem., 1999,(2):235−245. [19] Yang Weiguang, Wang Yali, Shi Weimin. One-step synthesis of single-crystal anatase TiO2 tetragonal faceted-nanorods for improved-performance dye-sensitized solar cells[J]. Cryst Eng Comm, 2012,14:230−234. doi: 10.1039/C1CE05844D -





 下载:
下载: