Study on resource utilization of by-products of chloride titanium dioxide
-
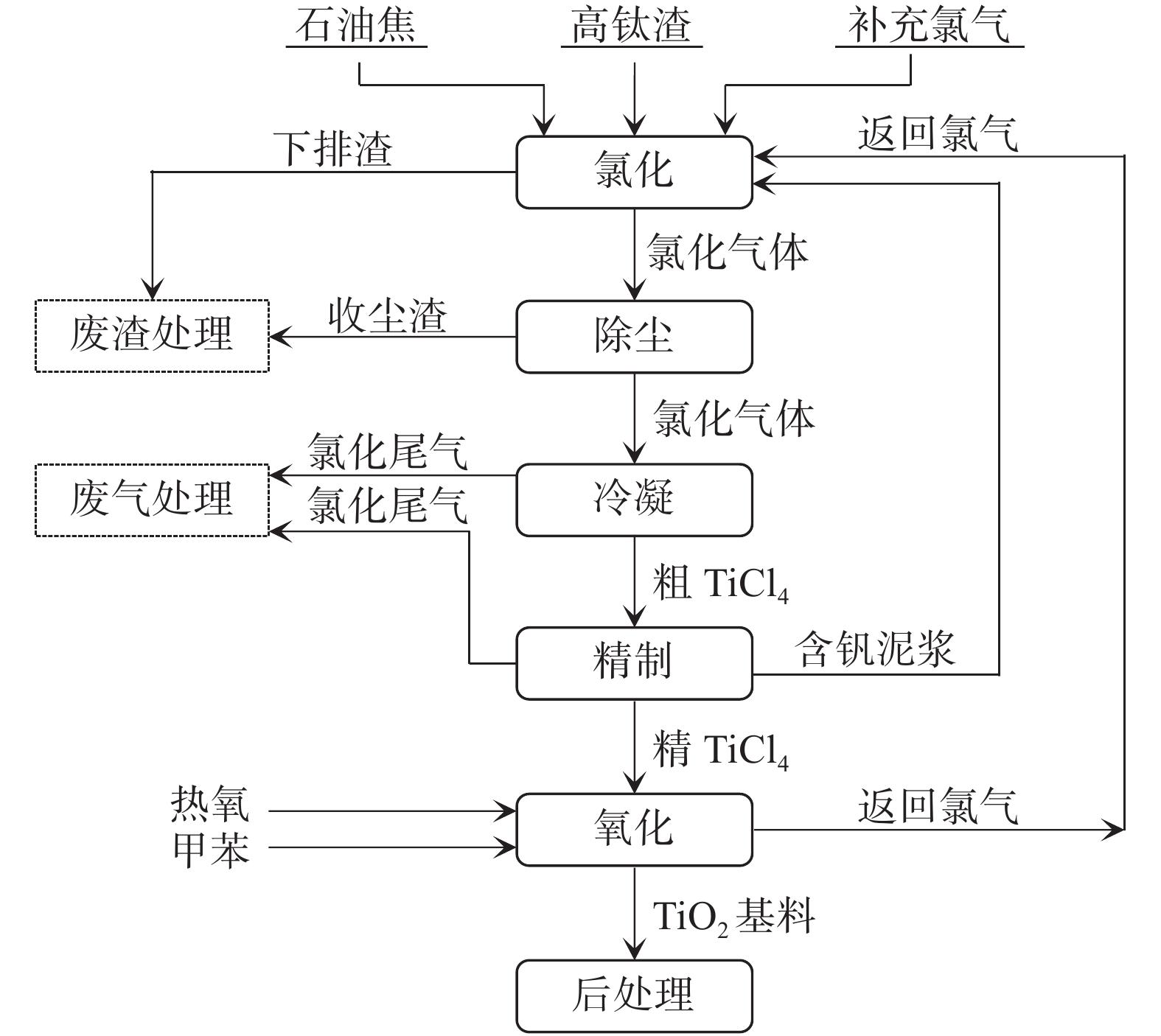
摘要: 以氯化法钛白粉生产过程中的副产物废盐酸和氯化尾气吸收液、工业氢氧化铝和七水硫酸亚铁为原料, 在双氧水催化氧化条件下, 制备得到一系列新型无机高分子混凝剂聚合氯化硫酸铝铁(PAFCS),其外观为红褐色透明液体, 体积密度1.39~1.43 g/cm3, 全铁与三氧化二铝(TFe+Al2O3)总含量≥10.5%, 可以用作水处理用混凝剂。与市售混凝剂相比,制备所得聚氯化硫酸铝铁对高化学需氧量(COD)的垃圾渗滤液生化尾水有较好的处理效果, COD去除率达到68.51%, 优于传统混凝剂聚合硫酸铁。高效聚合氯化硫酸铝铁的制备为我国氯化法钛白粉生产工艺中副产物的资源化利用提供了解决思路和可行方法。Abstract: In this paper, a series of new inorganic polymer coagulants (Polymeric Aluminum Ferric Chloride Sulfate, PAFCS) were prepared by using waste hydrochloric acid and chlorinated tail gas absorbing liquid from by-products of the production of titanium dioxide, in combination with industrial aluminum hydroxide and ferrous sulfate heptahydrate, under the conditions of hydrogen peroxide catalytic oxidation. The appearance of the prepared PAFCS was a reddish-brown transparent liquid with a density of 1.39 to 1.43 g/cm3, and the total content of iron and alumina (TFe + Al2O3) was ≥10.5%, which could be used as coagulant for water treatment. The PAFCS prepared in this paper were used to treat landfill leachate biochemical tail water, in which the removal rate of COD reached 68.51%, which was superior to the traditional coagulant polymeric ferric sulfate (PFS). At the same time, this work provides ideas for solving the problem of resource utilization and clean production of by-products in the production process of titanium dioxide by chlorination method in China.
-
表 1 不同类型PAFCS的物料组成
Table 1. Material compositions of different types of PAFCS
编号 n(Fe):n(Al2O3) 配比/ g 废盐酸 氢氧化铝 氯化尾气吸收液 七水硫酸亚铁 双氧水 P-1 6∶1 102 8 48 162 62 P-2 4∶1 82 8 32 108 41 P-3 2∶1 62 8 16 54 20 表 2 不同类型PAFCS的性能参数
Table 2. Performance parameters of the different types of PAFCS
序号 n(Fe)∶n(Al2O3) 全铁含量/% Al2O3含量/% 密度/ (g·cm−3) 盐基度/% pH值(1%水溶液) P-1 6∶1 8.77 1.48 1.43 11.21 2.57 P-2 4∶1 8.19 2.08 1.42 14.81 2.89 P-3 2∶1 6.82 3.46 1.39 15.62 3.12 表 3 自制PAFCS与市售聚合硫酸铁混凝剂处理垃圾渗滤液的效果试验
Table 3. Treatment of landfill leachate with PAFCS and commercial polyferric sulfate coagulant
投加量/(mg·L−1) 聚氯化硫酸铝铁(P-3) 聚合硫酸铁(P-0) 出水COD/(mg·L−1) 出水COD去除率/ % 出水COD/(mg·L−1) 出水COD去除率/ % 2 000 469.1 51.83 543.9 43.99 2200 449.2 53.93 506.5 47.91 2400 429.2 56.02 467.4 52.01 2600 310.2 68.51 474.4 51.28 2800 365.8 62.68 470.0 51.74 3000 406.6 58.39 491.4 49.19 -
[1] Liu Jun. Development status and future of titanium dioxide[J]. Jiangsu Salt Science & Technology, 2019,46(4):3−4. (刘军. 钛白粉发展现状与未来[J]. 现代盐化工, 2019,46(4):3−4. doi: 10.3969/j.issn.1005-880X.2019.04.002 [2] Gupta S M, Tripathi M. A review of TiO2 nanoparticles[J]. Chinese Science Bulletin, 2011,56(16):1639. doi: 10.1007/s11434-011-4476-1 [3] Liu Guangrong, Zhou Gaoming, Liu Xianghai, et al. Current situation and prospect of China’s titanium dioxide production in 2018[J]. Ferro-Alloys, 2019,50(4):45−48. (刘光蓉, 周高明, 刘祥海, 等. 2018年我国钛白粉生产现状及展望[J]. 铁合金, 2019,50(4):45−48. [4] Bi Sheng. Status and development of titanium dioxide industry in 2019[J]. Iron Steel Vanadium Titanium, 2019,40(4):1−3. (毕胜. 2019年中国钛白粉工业状况及发展趋势[J]. 钢铁钒钛, 2019,40(4):1−3. doi: 10.7513/j.issn.1004-7638.2019.04.001 [5] Ma Yanping, Liu Hongxing, He Benliu, et al. Research on production technology of titanium dioxide by chlorination[J]. Yunnan Chemical Technology, 2019,46(6):94−95, 98. (马艳萍, 刘红星, 和奔流, 等. 氯化法钛白粉的生产工艺探究[J]. 云南化工, 2019,46(6):94−95, 98. [6] Huang Songyi. A preliminary analysis of the comprehensive recycling utilization of the chlorination residue in the production of titanium dioxide by chlorination process[J]. Mechanical & Electrical Engineering Technology, 2019,48(8):180−182. (黄宋义. 浅谈氯化法钛白粉生产过程产生的氯化渣资源化综合利用[J]. 机电工程技术, 2019,48(8):180−182. doi: 10.3969/j.issn.1009-9492.2019.08.066 [7] Wu Hongji, Qu Shuling. The current status and prospects of titanium dioxide by chlorination process[J]. China Paint, 1996,(6):28−31. (吴鸿基, 屈树岭. 我国氯化法钛白的现状和前景[J]. 中国涂料, 1996,(6):28−31. [8] ZQUEZ G M J, BOL VAR J P, GARCIA-TENORIO R, et al. A review of the production cycle of titanium dioxide pigment[J]. Materials Sciences and Applications, 2014,5(7):441−458. doi: 10.4236/msa.2014.57048 [9] Dong Linhui, Li Yang, Zhou Xuefang, et al. Preparation of poly ferric-aluminous-sulphate(PAFS) and its performance in phosphorous removal[J]. Inorganic Chemicals Industry, 2019,51(5):57−60. (董林辉, 李杨, 周雪芳, 等. 聚合硫酸铁铝的制备及除磷性能研究[J]. 无机盐工业, 2019,51(5):57−60. [10] Zhang Zhi, Zhang Chao, Li Miao, et al. Preparation and stability mechanism of polyaluminum ferric sulfatochloride[J]. Ion Exchange and Adsorption, 2015,31(3):260−271. (张智, 张超, 李淼, 等. 聚合硫酸氯化铝铁合成及稳定性机理研究[J]. 离子交换与吸附, 2015,31(3):260−271. [11] Li Mingyu, Pan Qian, Wang Liyan, et al. Removal algae with different coagulants from micro-polluted water in Liuxi River[J]. China Environmental Science, 2010,(11):46−51. (李明玉, 潘倩, 王丽燕, 等. 不同混凝剂对流溪河水源水中藻类去除的对比[J]. 中国环境科学, 2010,(11):46−51. [12] Zhu G, Zheng H, Chen W, et al. Preparation of a composite coagulant: Polymeric aluminum ferric sulfate (PAFS) for wastewater treatment[J]. Desalination, 2012,(285):315−323. -





 下载:
下载: