Study on preparation of Fe/Fe2SiO4-based cermets by microwave in-situ reduction
-
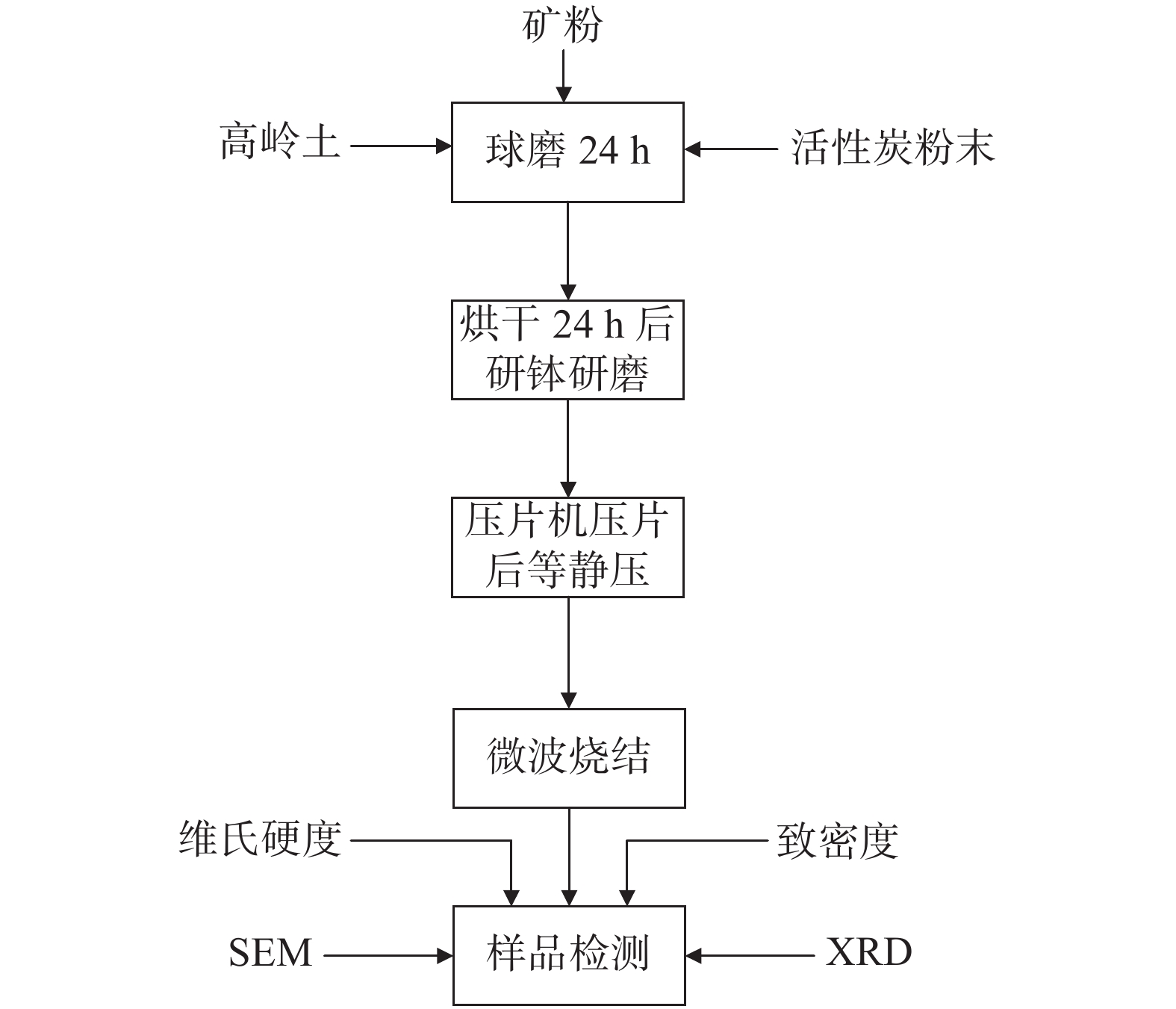
摘要: 以磁选铁精矿为主要原料,高岭土作为添加剂采用微波原位还原制备Fe/Fe2SiO4基金属陶瓷。概括介绍了利用微波加热原位还原磁选铁精矿研制铁基金属陶瓷的理论基础、主要技术路线和反应机理,结合XRD、SEM等分析测试方式,探究了高岭土添加含量对铁基金属陶瓷复合材料的物相、物理性能及微观组织的影响,为微波加热还原磁选铁精矿制备铁基多功能陶瓷材料的相关研究领域提供参考,对于促进矿产资源的高效综合利用具有重要的意义。结果表明:当烧结温度为835 ℃,保温时间为1 h,高岭土含量为15%时,制备的铁基金属陶瓷的综合性能最优,其主晶相为α-Fe,密度为5.56 g/cm3,维氏硬度值为7.35 GPa。Abstract: Fe/Fe2SiO4-based cermets were prepared with magnetic separation iron concentrate as the main raw material and kaolin as the additive by means of microwave in-situ reduction. The theoretical basis, main technical route, and reaction mechanism of iron-based cermets developed by using microwave heating in situ reductions of magnetic separation iron concentrates are summarized. Combined with XRD, SEM, and other analysis and testing methods, the effect of kaolin content on phase, physical properties, and microstructure of iron-based cermets composites had been explored. The results show that under conditions of Kaolin content at 15%, sintering at 835 ℃ for 1 hour can make iron-based cermets composites achieve the best comprehensive properties, where dominant phase is α-Fe, its density is 5.56 g/cm3, and Vickers hardness is 7.35 GPa.
-
Key words:
- Fe/Fe2SiO4-based cermet /
- iron concentrate /
- Kaolin /
- microwave heating /
- in-situ reduction /
- density /
- Vickers hardness
-
表 1 磁选铁精矿的化学成分
Table 1. The chemical compositions of iron concentrate by magnetic separation
% TFe FeO SiO2 Al2O3 CaO MgO MnO2 TiO2 65.50 27.90 2.47 0.047 0.76 0.88 1.01 0.029 Na2O S Nb2O5 K2O BaO P F 0.25 1.08 0.073 0.1 0.036 0.069 0.28 表 2 试样配比
Table 2. Sample ratio
编号 原料/g 高岭土/% C1 50 5 C2 50 10 C3 50 15 C4 50 20 注:原料配比为矿粉∶活性炭=17∶3。 -
[1] Wu Qiujie, Lu Zhenfu, Cao Jincheng. Study on the status quo of the development and utilization of my country’s large-scale iron ore resource base[J]. Modern Mining, 2020,36(8):113−115, 138. (武秋杰, 吕振福, 曹进成. 我国铁矿大型资源基地开发利用现状研究[J]. 现代矿业, 2020,36(8):113−115, 138. doi: 10.3969/j.issn.1674-6082.2020.08.034 [2] Luo Xiaoli. The status quo of my country’s iron ore resources exploration and development and countermeasures[J]. Modern Mining, 2019,35(12):28−32. (罗小利. 我国铁矿资源勘查开发现状及对策建议[J]. 现代矿业, 2019,35(12):28−32. doi: 10.3969/j.issn.1674-6082.2019.12.008 [3] Jiang Xuewei. Analysis of the development status and prospects of China’s iron ore industry[J]. China Metal Bulletin, 2017,(7):160−161. (姜雪薇. 中国铁矿行业发展现状及前景分析[J]. 中国金属通报, 2017,(7):160−161. [4] Chen Min, Xiao Xuan, Tang Aitao. Study on Fe-TiCN cermet prepared by titanium concentrate[J]. Non-Ferrous Metal Science and Engineering, 2015,6(5):69−72, 134. (陈敏, 肖玄, 汤爱涛. 钛精矿制备Fe-TiCN金属陶瓷的研究[J]. 有色金属科学与工程, 2015,6(5):69−72, 134. [5] Yin Xueliang, Ma Heli, Liu Yang, et al. Preparation of dense CA2-CA6 composites from bauxite and limestone ore[J]. Refractories, 2020,54(4):326−329. (尹雪亮, 马贺利, 刘洋, 等. 铝土矿、石灰石矿制备致密CA2-CA6复合材料[J]. 耐火材料, 2020,54(4):326−329. doi: 10.3969/j.issn.1001-1935.2020.04.012 [6] (马子钧. 利用硅酸盐工业废(尾)矿制备发泡陶瓷的研究[D]. 北京: 北京工业大学, 2019.)Ma Zijun. Study on the preparation of foamed ceramics using silicate industrial waste (tailing) ore[D]. Beijing: Beijing University of Technology, 2019. [7] Zhou Shuzhu, Wu Xiaobo, Gao Lingyan, et al. Research progress and industrial application status of microwave sintering of ceramic materials[J]. Cemented Carbide, 2012,29(3):174−181. (周书助, 伍小波, 高凌燕, 等. 陶瓷材料微波烧结研究进展与工业应用现状[J]. 硬质合金, 2012,29(3):174−181. doi: 10.3969/j.issn.1003-7292.2012.03.009 [8] Cui Lisheng, Han Yuexin. Application of microwave technology in mining industry[J]. Nonferrous Mining and Metallurgy, 2005,(S1):54−55, 57. (崔礼生, 韩跃新. 微波技术在矿业中的应用[J]. 有色矿冶, 2005,(S1):54−55, 57. [9] Schyuy S A. Application of microwave in mining[J]. Mining Engineering, 2003,(6):14−18. (施尤伊S A. 微波在矿业中的应用[J]. 矿业工程, 2003,(6):14−18. [10] Lin Jing, Su Jie, Peng Jinhui, et al. Application status and prospects of microwave technology in the field of metallurgical smelting[J]. Vacuum Electronic Technology, 2016,(6):36−42. (蔺琎, 苏杰, 彭金辉, 等. 微波技术在冶金熔炼领域应用现状及前景[J]. 真空电子技术, 2016,(6):36−42. [11] Li Lei, Zhu Hongbo, Zhang Libo, et al. Extended research on microwave carbothermic reduction of ilmenite[J]. Materials Review, 2015,29(10):124−127. (李磊, 朱红波, 张利波, 等. 微波碳热还原钛铁矿扩试研究[J]. 材料导报, 2015,29(10):124−127. [12] Guo Yufeng, You Gao, Jiang Tao, et al. Solid state reduction behavior of Panzhihua ilmenite[J]. Journal of Central South University (Natural Science Edition), 2010,41(5):1639−1644. (郭宇峰, 游高, 姜涛, 等. 攀枝花钛铁矿固态还原行为[J]. 中南大学学报(自然科学版), 2010,41(5):1639−1644. [13] Fahamsyah H Latief, Naser A Alsaleh, Nashmi Alrasheedi, et al. Effects of oxidation and alumina addition on the physical and mechanical properties of Ti/ Al2O3 composites prepared by semi-powder metallurgy method[J]. Oxidation of Metals, 2019,92(5-6):561−572. doi: 10.1007/s11085-019-09923-z [14] Sharifitabar M. On the formation of Al2O3 nanofibers during self-propagating high-temperature synthesis of TiO2–Al–C system in various environments[J]. Ceramics International, 2020,46(10):603. [15] Tian Zhihao, Wu Xiaobo, Gao Pingping, et al. Research progress on toughening of Ti(C, N)-based cermets[J]. Cemented Carbide, 2019,36(4):313−320. (田智豪, 伍小波, 高平平, 等. Ti(C, N)基金属陶瓷增韧研究进展[J]. 硬质合金, 2019,36(4):313−320. [16] Zhao Yonghong, Zimmerman M, Kohlstedt D L. Experimental study on high temperature deformation of iron-rich olivine[J]. Acta Petrologica Sinica, 2005,(3):999−1004. (赵永红, Zimmerman M, Kohlstedt D L. 富铁橄榄石的高温变形实验研究[J]. 岩石学报, 2005,(3):999−1004. doi: 10.3321/j.issn:1000-0569.2005.03.038 [17] Bulin Chaoke, Guo Ting, Zhang Bangwen, et al. Analysis of the origin of Fe2O3 stepwise reduction sequence[J]. Metal Mine, 2013,(6):53−57, 92. (布林朝克, 郭婷, 张邦文, 等. Fe2O3逐级还原顺序起源分析[J]. 金属矿山, 2013,(6):53−57, 92. doi: 10.3969/j.issn.1001-1250.2013.06.016 [18] (王筱留. 钢铁冶金学(炼铁部分)[M]. 北京: 冶金工业出版社, 2007: 79−96.)Wang Xiaoliu. Iron and steel metallurgy (Ironmaking part) [M]. Beijing: Metallurgical Industry Press, 2007: 79−96. [19] Li Xiaobin, Wang Hongyang, Zhou Qiusheng, et al. Reaction behavior of kaolinite with ferric oxide during reduction roasting[J]. Transactions of Nonferrous Metals Society of China, 2019,29(1):93. [20] Zhou Qiusheng, Li Chuang, Li Xiaobin, et al. Reaction behavior of Fe2O3 during reduction sintering of Fe2O3-SiO2-Al2O3 system[J]. Transactions of Nonferrous Metals Society of China, 2016,26(3):842−848. doi: 10.1016/S1003-6326(16)64175-4 [21] BARIN I. Thermochemical data of pure substances [M]. Weinheim: VCH Verlagsgesellschaft mbH, 1995. [22] Mei Xiangong, Yuan Mingliang, Chen Yin. A preliminary study on the effect of alkaline oxides in the direct reduction process of a high-iron red peat-based coal[J]. Comprehensive Utilization of Mineral Resources, 1995,(2):1−5. (梅贤恭, 袁明亮, 陈荩. 某高铁赤泥煤基直接还原过程中的碱性氧化物效应初探[J]. 矿产综合利用, 1995,(2):1−5. [23] Han Yuexin, Sun Yongsheng, Li Yanfeng, et al. Evolution of phase and structure of oolitic hematite deep reduction[J]. Journal of Iron and Steel Research, 2019,31(2):95−102. (韩跃新, 孙永升, 栗艳锋, 等. 鲕状赤铁矿深度还原物相及结构的演化规律[J]. 钢铁研究学报, 2019,31(2):95−102. -





 下载:
下载: