Simultaneous preparation of calcium titanate from ilmenite by direct reduction and recovery of iron
-
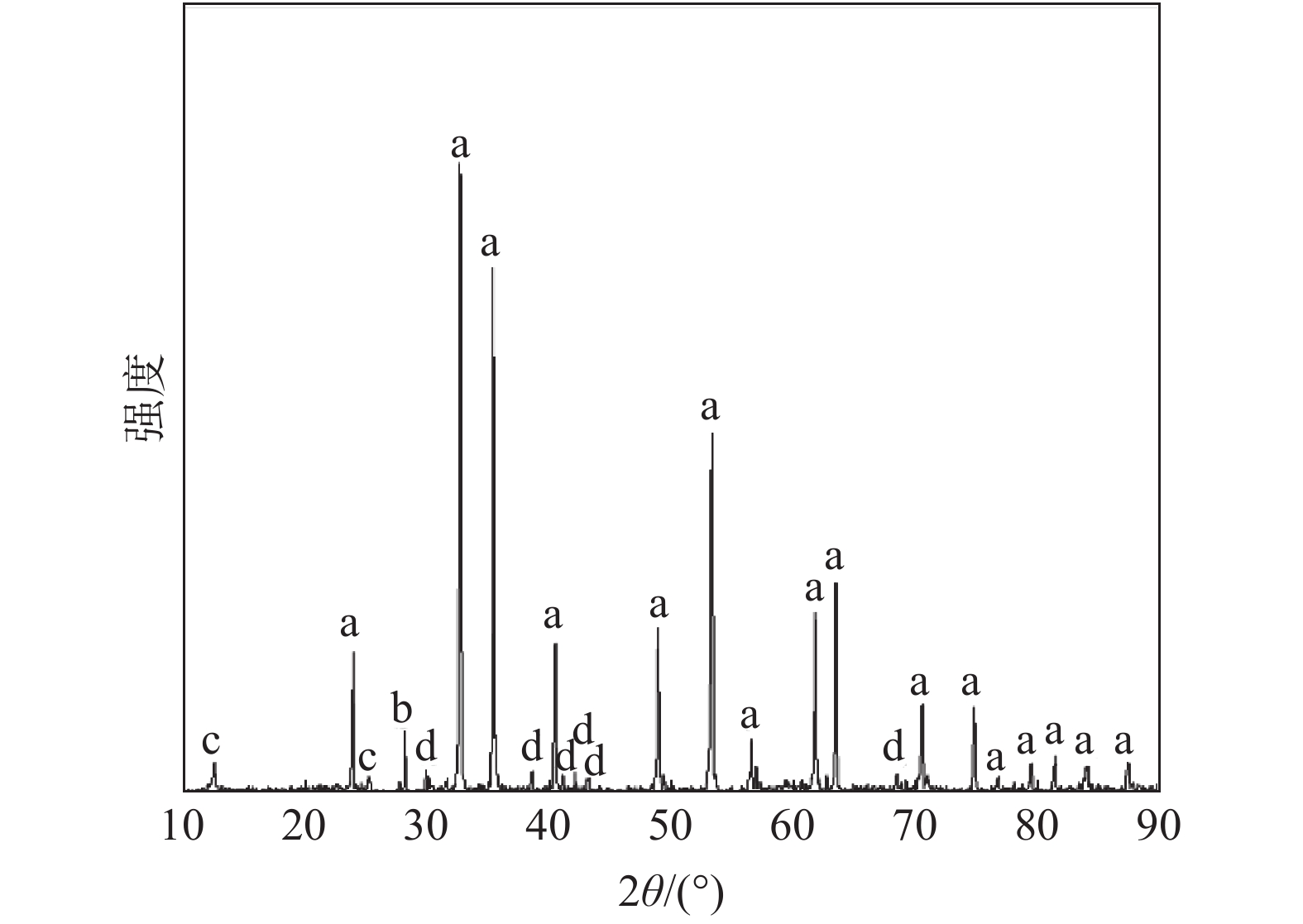
摘要: 研究了钛铁矿煤基包埋法直接还原铁同步制备钛酸钙过程中钛的物相转化和钛酸钙的生成机制,阐明了添加剂碳酸钙用量和焙烧温度等因素对还原铁和钛酸钙的影响规律和作用机理。结果表明,含60%碳酸钙的钛铁矿生球团在1400 ℃下煤基包埋法恒温焙烧180 min,可以在制得直接还原铁的同时使钛以纯钛酸钙的形式产出。在添加剂碳酸钙的作用下,焙烧温度>1300 ℃时,钛酸钙开始作为主要含钛物相产出,试验得出的最佳焙烧温度为1400 ℃。随着碳酸钙用量的增加,焙烧产物中黑钛石的含量逐渐减少,钛酸钙的含量逐渐增多。但是碳酸钙用量大会导致还原铁颗粒变细,不利于后续的磨矿磁选分离。实验室条件下通过两段磨矿磁选最终获得还原铁产品TFe品位为81.86%、回收率为91.27%,钛酸钙产品中Ti品位为26.95%、钛酸钙含量为76.37%,钛的回收率为90.15%。Abstract: The paper studied the phase transformation of titanium and the formation mechanism of calcium titanate during the simultaneous preparation of calcium titanate by direct reduction of iron from ilmenite coal-based embedding method, and clarified the influence of additives such as calcium carbonate dosage and roasting temperature on reduced iron and titanium. The influence law and mechanism of calcium acid. The results show that the raw ilmenite pellets containing 60% calcium carbonate are roasted at a constant temperature of 1400 ℃ for 180 min by the coal-based embedding method, which can produce direct reduced iron while simultaneously producing titanium in the form of pure calcium titanate. Under the action of the additive calcium carbonate, when the calcination temperature is >1300 ℃, calcium titanate begins to be produced as the main titanium-containing phase, and the best calcination temperature obtained by the experiment is 1400 ℃. As the amount of calcium carbonate increases, the content of black titanite in the calcined product gradually decreases, and the content of calcium titanate gradually increases. However, the large amount of calcium carbonate causes the reduced iron particles to become finer, which is not conducive to the subsequent grinding and magnetic separation. Under laboratory conditions, the TFe grade of the reduced iron product is 81.86% and the recovery rate is 91.27%. The grade of Ti in the calcium titanate product is 26.95%, the content of calcium titanate is 76.37% and the recovery rate of titanium is 90.15%.
-
Key words:
- ilmenite /
- direct reduction /
- coal-based embedding method /
- roasting /
- calcium titanate /
- reduced iron
-
表 1 试样粒度组成
Table 1. Grain size composition of samples
粒级/mm 含量/% +0.074 20.28 −0.074~+0.045 31.19 −0.045~+0.038 32.98 −0.038 15.55 合计 100 表 2 试样的主要化学组成
Table 2. Main chemical compositions of samples
% TiO2 TFe MgO SiO2 Al2O3 MnO CaO S V2O5 45.28 29.01 4.91 2.24 0.89 0.610 0.55 0.15 0.058 -
[1] Zhang Tao, Song Bing. Research status of preparation of artificial rutile from iron and titanium by reduction grinding separation of ilmenite[J]. Light Metals, 2020,(502):42−44. (张涛, 宋兵. 钛铁矿还原-磨选法分离铁钛制备人造金红石研究现状[J]. 轻金属, 2020,(502):42−44. [2] Guo Yufeng, You Gao, Jiang Tao, et al. Solid state reduction behavior of Panzhihua ilmenite[J]. Journal of Central South University (Natural Science Edition), 2010,41(195):1639−1644. (郭宇峰, 游高, 姜涛, 等. 攀枝花钛铁矿固态还原行为[J]. 中南大学学报(自然科学版), 2010,41(195):1639−1644. [3] Wu Shichao, Sun Tichang, Yang Huifen. Study on phosphorus removal of high-phosphorus oolitic hematite abroad by direct reduction and magnetic separation[J]. Metal Mines, 2019,48(11):109−114. (吴世超, 孙体昌, 杨慧芬. 国外某高磷鲕状赤铁矿直接还原-磁选降磷研究[J]. 金属矿山, 2019,48(11):109−114. [4] (陈江安. 低品位褐铁矿石煤基直接还原过程矿物转化规律及机理[D]. 北京: 北京科技大学, 2021.)Chen Jiang, an. Mineral transformation law and mechanism in coal-based direct reduction process of low-grade limonite[D]. Beijing: University of Science and Technology Beijing, 2021. [5] Zhang Jun, Xu Haichuan, Wang Feng, et al. New process for utilization of sea sand vanadium-bearing titanomagnetite[J]. Iron Steel Vanadium Titanium, 2021,42(2):5−9. (张俊, 许海川, 王锋, 等. 海砂钒钛磁铁矿高效综合利用新工艺[J]. 钢铁钒钛, 2021,42(2):5−9. [6] Xie Zhicheng, Hu Bing, Hu Peiwei. Research on new technology of high-efficient comprehensive utilization of vanadium-titanium magnetite[J]. Iron Steel Vanadium Titanium, 2020,41(5):14−21. (谢志诚, 胡兵, 胡佩伟. 钒钛磁铁矿高效综合利用新工艺研究[J]. 钢铁钒钛, 2020,41(5):14−21. [7] Shen Fengman, Jiang Xin, Gao Jianqiang, et al. Situation and prospect on production technology of reduction iron[J]. Iron and Steel, 2017,52(1):7−12. (沈峰满, 姜鑫, 高强健, 等. 直接还原铁生产技术的现状及展望[J]. 钢铁, 2017,52(1):7−12. [8] (柳政根, 储满生, 唐珏, 等. 特色冶金资源煤基直接还原-磁选分离研究[C]//2014年全国炼铁生产技术会暨炼铁学术年会文集(下) .郑州: 中国金属学会, 2014: 413−420.)Liu Zhenggen, Chu Mansheng, Tang Jue, et al. Research on coal-based direct reduction magnetic separation for characteristic metallurgical resources[C]// Proceedings of 2014 National Ironmaking Production Technology Conference and Annual Ironmaking Academic Conference (Part 2). Zhengzhou: The Chinese Society for Metals, 2014: 413−420. [9] Li Xiaohui, Kou Jue, Sun Tichang, et al. Effect of direct reduction time of vanadium titanomagnetite concentrate on the preparation and photocatalytic performance of calcium titanate[J]. Physicochemical Problems of Mineral Processing, 2020,57(1):75−86. doi: 10.37190/ppmp/128684 [10] Chen Chao, Sun Tichang, Geng Chao, et al. Formation and mechanism of magnesium titanate in the process of ilmenite reduction[J]. Mineral Processing and Extractive Metallurgy Review, 2019,(2):1−10. [11] Li Xiaohui, Kou Jue, Sun Tichang, et al. Coal and coke based reduction of vanadium titanomagenetite concentrate by the addition of calcium carbonate[J]. Mineral Processing and Extractive Metallurgy Review, 2021,42(2):115−122. doi: 10.1080/08827508.2019.1702039 [12] Li Xiaohui, Kou Jue, Sun Tichang, et al. Formation of calcium titanate in the carbothermic reduction of vanadium titanomagnetite concentrate by adding CaCO3[J]. International Journal of Minerals Metallurgy and Materials, 2020,27(6):745−753. doi: 10.1007/s12613-019-1903-9 [13] (陈超. 钒钛磁铁矿直接还原过程中钛酸镁生成及机理研究[D]. 北京: 北京科技大学, 2019.)Chen Chao. Study on the formation and mechanism of magnesium titanate during the direct reduction of vanadium titanomagnetite[D]. Beijing: University of Science and Technology Beijing, 2019. [14] (赵鸿宇. 形状和尺寸可控的钛酸钙颗粒的制备与表征[D]. 兰州: 兰州大学, 2013.)Zhao Hongyu. Synthesis and characterization of CaTiO3 particles with controlled shape and size[D]. Lanzhou: Lanzhou University, 2013. [15] Zhu Haikui, Liu Min, Zhou Hongqing. Effect of additives on CaTiO3 effect of ceramic properties[J]. Electronic Components and Materials, 2005,24(7):38−40. (朱海奎, 刘敏, 周洪庆. 添加剂对CaTiO3陶瓷性能的影响[J]. 电子元件与材料, 2005,24(7):38−40. doi: 10.3969/j.issn.1001-2028.2005.07.011 [16] Peng Mengni, Yan Zhiguo, Yin Xia, et al. Progress in perovskite oxide catalysis[J]. Applied Chemical Industry, 2020,46(9):212−216. (彭梦妮, 闫志国, 殷霞, 等. 钙钛矿氧化物催化研究进展[J]. 应用化工, 2020,46(9):212−216. [17] (张红杰. 钛酸钙光催化剂掺杂改性及电子结构的研究[D]. 哈尔滨: 哈尔滨工业大学, 2010.)Zhang Hongjie. Study on doping of calcium titanate photocatalyst and its electronic structure[D]. Harbin: Harbin University of Technology, 2010. [18] Han Chong, Yang He, Xue Xiangxin. Photocatalytic property of the solid-phase synthsized titanate calcium[J]. Environmental Chemistry, 2010,29(5):831−834. (韩冲, 杨合, 薛向欣. 固相合成钛酸钙的光催化性能[J]. 环境化学, 2010,29(5):831−834. [19] Zeng Pujun, Yu Liping, Qiu Zhongxian, et al. Significant enhancement of luminescence intensity of CaTiO3: Eu3+ red phosphor prepared by sol-gel method and co-doped with Bi3+ and Mg2+[J]. Journal of Sol-gel Science and Technology, 2012,64(2):315−323. doi: 10.1007/s10971-012-2860-1 [20] Gao Qiang, Meng Jie, Yang Ya, et al. Zirconium doping in calcium titanate perovskite oxides with surface nanostep structure for promoting photocatalytic hydrogen evolution[J]. Applied Surface Science, 2021,542:148544. doi: 10.1016/j.apsusc.2020.148544 [21] Singh D K, Manam J. Structural and luminescence properties of CaTiO3: Eu3+ phosphor synthesized by chemical co-precipitation method for the application of solid state lighting devices[C]//AIP Conference Proceedings. AIP Publishing LLC, 2016. [22] Chen Wanbing, Zhang Shaowei, Wang Zhoufu, et al. Fabrication of CaTiO3 powder by molten salt synthesis[J]. Journal of Wuhan University of Science and Technology (Natural Science Edition), 2007,117(6):581−583, 591. (陈万兵, 张少伟, 王周福, 等. 熔盐合成法制备CaTiO3粉体的研究[J]. 武汉科技大学学报(自然科学版), 2007,117(6):581−583, 591. [23] Hong Bingxin, Fu Wenzhang. Mineralogical characteristics of anosovite solid solution[J]. Comprehensive Utilization of Minerals, 2012,175(3):55−58, 66. (洪秉信, 傅文章. 黑钛石固溶体的矿物学特征[J]. 矿产综合利用, 2012,175(3):55−58, 66. doi: 10.3969/j.issn.1000-6532.2012.03.015 [24] (杨绍利, 盛继孚. 钛铁矿熔炼钛渣与生铁技术[M]. 北京: 冶金工业出版社, 2006: 196−197.)Yang Shaoli, Sheng Jifu. Ilmenite smelting titanium slag and pig iron technology[M]. Beijing: Metallurgical Industry Press, 2006: 196−197. [25] Wang Shuai, Guo Yufeng, Zheng Fuqiang, et al. Behavior of vanadium during reduction and smelting of vanadium titanomagnetite metallized pellets[J]. Transactions of Nonferrous Metals Society of China, 2020,30(6):1687−1696. doi: 10.1016/S1003-6326(20)65330-4 [26] (李小辉. 钒钛磁铁矿精矿直接还原回收铁同步生成钛酸钙的研究[D]. 北京: 北京科技大学, 2021.)Li Xiaohui. Study on recovering iron and simultaneous forming calcium titanate by direct reduction from vanadium titanomagnetite concentrate[D]. Beijing: Beijing University of science and technology, 2021. -





 下载:
下载: