Effect of SO2 on adsorption of NO from sintering flue gas by modified activated carbon
-
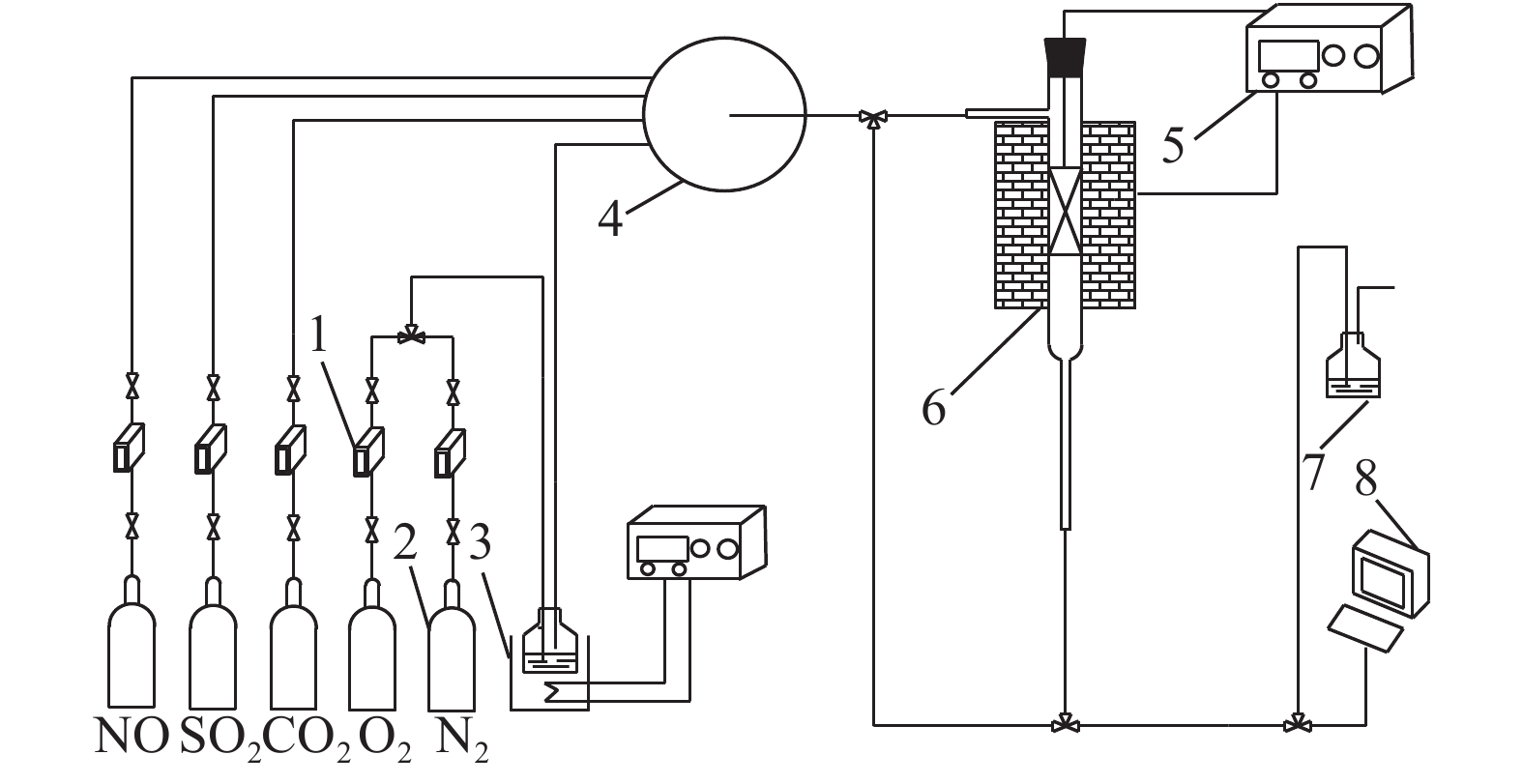
摘要: 为探究SO2对改性活性炭吸附法去除烧结烟气中NO效果的影响,减少NOx的排放,降低钢铁行业对环境的污染,采用碳酸钾(K2CO3)浸渍改性的方法制备了改性活性炭吸附剂,并考察了不同浓度SO2对改性活性炭吸附烧结烟气中NO的影响,及SO2对改性活性炭循环吸附脱附性能的影响。结果表明:SO2能够提高改性活性炭初始NO吸附速率,但显著降低NO的饱和吸附容量;SO2通过化学吸附方式占据活性位点,形成的亚硫酸盐及硫酸盐加热难以脱附,造成改性活性炭难以恢复初始吸附容量;SO2影响下改性活性炭吸附容量的衰减速率约为1.41417 mg/g,可以根据衰减速率,配合移动床吸附装置,选择合适的新鲜活性炭投入参数,进而指导改性活性炭吸附去除烧结烟气中NO的应用,有效降低环境污染,实现资源的高效利用。Abstract: In order to reduce NOx emission and alleviate the environmental pollution from steel industry, a modified activated carbon adsorbent was prepared by impregnation with potassium carbonate (K2CO3). The effect of SO2 concentration on the adsorption of NO in sintering flue gas by the modified activated carbon and the cyclic adsorption and desorption performance of the modified activated carbon were investigated. The results show that SO2 can improve the initial adsorption rate of NO but significantly reduce the saturated adsorption capacity of the modified activated carbon. SO2 occupies the active sites of the carbon by chemical adsorption, and the sulfites and sulfates formed are difficult to be desorbed by heating, resulting in difficulty of recovering the initial adsorption capacity of the modified activated carbon. Under the influence of SO2, the attenuation rate of the adsorption capacity of the modified activated carbon is about 1.414 17 mg/g. According to the attenuation rate and the moving bed adsorption device, appropriate input parameters of the fresh activated carbon can be adopted to guide the application of the modified activated carbon for removal of NO in the sintering flue gas, which can effectively reduce the environmental pollution and realize effficient utilization of resources.
-
Key words:
- sintering /
- flue gas /
- nitric oxide /
- modified activated carbon /
- adsorption /
- sulfur dioxide
-
表 1 不同SO2浓度下改性活性炭NO吸附容量及NO初始吸附速率
Table 1. Adsorption capacity and initial adsorption rate of NO by modified activated carbon at different SO2 concentrations
SO2浓度/
(mg·m−3)NO吸附量/
(mg·g−1)NO初始吸附速率/
(mg·min−1)0 16.576 0.412 30 13.078 0.494 70 10.193 0.485 715 7.589 0.471 表 2 不同循环次数下改性活性炭NO吸附容量
Table 2. Adsorption capacity of NO under different cycles by modified activated carbon
吸附次数 NO吸附量/(mg·g−1) 第一次 15.461 第二次 14.281 第三次 11.814 第四次 10.679 第五次 9.532 第六次 7.141 第七次 6.127 第八次 5.790 第九次 4.344 -
[1] (都亚茹. 改性活性碳纤维吸附氮氧化物的实验研究[D]. 太原: 太原理工大学, 2019.)Du Yaru. Experimental study on adsorption of nitrogen oxides by modified activated carbon fibers[D]. Taiyuan: Taiyuan University of Technology, 2019. [2] Wang Peng, Wang Xuehai, Wang Kuanling, et al. Research development of new type metal organic framework catalysts for the selective catalytic reduction of NOx[J]. Industrial Catalysis, 2019,27(5):1−4. (汪鹏, 王学海, 王宽岭, 等. 新型金属有机骨架材料催化剂选择性催化还原氮氧化物研究进展[J]. 工业催化, 2019,27(5):1−4. doi: 10.3969/j.issn.1008-1143.2019.05.001 [3] Mochida I, Korai Y, Shirahama M, et al. Removal of SOx and NOx over activated carbon fibers[J]. Carbon, 2000,38(2):227−239. doi: 10.1016/S0008-6223(99)00179-7 [4] Izquierdo M T, Rubio B, Mayoral C, et al. Low cost coal-based carbons for combined SO2 and NO removal from exhaust gas[J]. Fuel, 2003,82(2):147−151. doi: 10.1016/S0016-2361(02)00249-1 [5] Huang Bangfu, Geng Chaoyang, Shi Zhe, et al. Influence factors and preparation conditions of Ni/AC used as a low-temperature desulfurizer[J]. Ecology and Environment, 2018,27(1):108−114. (黄帮福, 耿朝阳, 施哲, 等. 载镍活性炭低温脱硫及其制备影响因素研究[J]. 生态环境学报, 2018,27(1):108−114. [6] Zhao Simeng, Huang Bangfu, Liu Lanpeng, et al. Study on adsorption properties of coconut shell activated carbon modified by HNO3 and γ-Fe2O3[J]. Powder Metallurgy Technology, 2020,38(2):121−125. (赵思孟, 黄帮福, 刘兰鹏, 等. HNO3和γ-Fe2O3改性椰壳活性炭吸附性能研究[J]. 粉末冶金技术, 2020,38(2):121−125. [7] Li X Q, Zhang L, Yang Z Q, et al. Hydrophobic modified activated carbon using PDMS for the adsorption of VOCs in humid condition[J]. Separation and Purification Technology, 2020,239:116517. doi: 10.1016/j.seppur.2020.116517 [8] Ma L, He M Y, Fu P B, et al. Adsorption of volatile organic compounds on modified spherical activated carbon in a new cyclonic fluidized bed[J]. Separation and Purification Technology, 2020,235:116146. doi: 10.1016/j.seppur.2019.116146 [9] Li Xia, Chen Sili, Zhuo Qiongfang, et al. On the adsorptive performance of carbaryl onto the activated carbons with the thermal treatment[J]. Journal of Safety and Environment, 2017,17(5):1915−1921. (李霞, 陈思莉, 卓琼芳, 等. 热改性活性炭吸附甲萘威的性能[J]. 安全与环境学报, 2017,17(5):1915−1921. [10] Liu G F, Li X C, Campos L C. Role of the functional groups in the adsorption of bisphenol A onto activated carbon: Thermal modification and mechanism[J]. Journal of Water Supply: Research and Technology - Aqua, 2017,66(2):105−115. doi: 10.2166/aqua.2017.047 [11] Xiao Chengyuan, Zhang Wenli, Lin Haibo, et al. Modification of a rice husk-based activated carbon by thermal treatment and its effect on its electrochemical performance as a supercapacitor electrode[J]. New Carbon Materials, 2019,34(4):341−348. (肖程元, 张文礼, 林海波, 等. 稻壳基活性炭的热处理改性及其电化学性能(英文)[J]. 新型炭材料, 2019,34(4):341−348. doi: 10.1016/S1872-5805(19)30021-6 [12] Shi Yan, Kong Zheng, Hu Changqing, et al. Microwave combined modified activated carbon for treatment of flue gas desulfurization efficiency of different concentrations of SO2[J]. Iron Steel Vanadium Titanium, 2019,40(5):84−88. (石焱, 孔征, 胡长庆, 等. 微波联合改性活性炭处理不同SO2浓度烟气脱硫效率[J]. 钢铁钒钛, 2019,40(5):84−88. [13] Qiu W J, Dou K, Zhou Y, et al. Hierarchical pore structure of activated carbon fabricated by CO2/microwave for volatile organic compounds adsorption[J]. Chinese Journal of Chemical Engineering, 2018,26(1):81−88. doi: 10.1016/j.cjche.2017.04.006 [14] Wang L, Chen Z Z, Wen H, et al. Microwave assisted modification of activated carbons by organic acid ammoniums activation for enhanced adsorption of acid red 18[J]. Powder Technology, 2018,323(1):230−237. [15] Yang Kun, Liu Yang, Yang Jing. Advances in the preparation and modification of activated carbon adsorption VOCs[J]. Guangdong Chemical Industry, 2018,45(1):87−89. (杨坤, 刘洋, 杨静. 制备及改性活性炭对 VOCs 吸附的研究进展[J]. 广东化工, 2018,45(1):87−89. doi: 10.3969/j.issn.1007-1865.2018.01.041 [16] Ebrahimi B, Mohammadiazar S, Ardalan S. New modified carbon based solid phase extraction sorbent prepared from wild cherry stone as natural raw material for the pre-concentration and determination of trace amounts of copper in food samples[J]. Microchemical Journal, 2019,147:666−673. doi: 10.1016/j.microc.2019.03.062 [17] Zhan Xiaocui, Kuang Wenjun, Ding Ding, et al. Effect of ultrasonic modification on catalytic ozonation of phenol over a Mn/AC catalyst[J]. Modern Chemical Industry, 2019,39(2):103−107. (占小翠, 旷文君, 丁丁, 等. 超声波改性强化Mn/AC催化臭氧化降解苯酚效能分析[J]. 现代化工, 2019,39(2):103−107. [18] Jauto A H, Memon S A, Channa A, et al. Efficient removal of cyanide from industrial effluent using acid treated modified surface activated carbon[J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2019,41(22):2715−2724. doi: 10.1080/15567036.2019.1568643 [19] Rashid U S, Bezbaruah A N. Citric acid modified granular activated carbon for enhanced defluoridation[J]. Chemosphere, 2020,252:126639. doi: 10.1016/j.chemosphere.2020.126639 [20] Su P D, Zhang J K, Tang J W, et al. Preparation of nitric acid modified powder activated carbon to remove trace amount of Ni(II) in aqueous solution[J]. Water Science and Technology: Journal of the International Association on Water Pollution Research, 2019,80(1):86−97. doi: 10.2166/wst.2019.248 [21] Chen Mingyan, Chen Jie, Liu Yucheng, et al. Desulfurization performance of activated carbon fibers modified by supported metal[J]. Environmental Protection of Chemical Industry, 2018,38(6):728−732. (陈明燕, 陈洁, 刘宇程, 等. 负载金属改性活性炭纤维的脱硫性能[J]. 化工环保, 2018,38(6):728−732. doi: 10.3969/j.issn.1006-1878.2018.06.018 [22] Sharififard H, Pepe F, Aprea P, et al. Chemical modification of activated carbon surface with iron functional groups for efficient separation of vanadium: Batch and column study[J]. Research on Chemical Intermediates, 2017,43(11):6553−6570. doi: 10.1007/s11164-017-3004-6 [23] Zhang Wenliang, Qin Fanfan, Fan Xing, et al. Research progress on preparation and application technology of activated carbon loaded metal[J]. Shandong Chemical Industry, 2019,48(20):80−81. (张文亮, 秦凡凡, 樊星, 等. 金属负载活性炭的制备与应用技术研究进展[J]. 山东化工, 2019,48(20):80−81. doi: 10.3969/j.issn.1008-021X.2019.20.030 [24] Hou Jianfeng, Wang Zhaowen, Li Tuofu, et al. High temperature modification on coconut shell activated carbon and adsorptivity of the activated carbon for K+ in aluminum electrolyte[J]. Journal of Northeastern University (Natural Science), 2016,37(12):1740−1744. (侯剑峰, 王兆文, 李拓夫, 等. 椰壳类活性炭高温改性及吸附铝电解质熔盐中 K+ 的性能[J]. 东北大学学报 (自然科学版), 2016,37(12):1740−1744. [25] Huang Bangfu, Geng Chaoyang, Shi Zhe, et al. Desulfurizing property of activated carbon modified by acid and alkali and nickel[J]. Bulletin of the Chinese Ceramic Society, 2018,(11):42. (黄帮福, 耿朝阳, 施哲, 等. 酸碱改性及负载镍对活性炭脱硫性能影响研究[J]. 硅酸盐通报, 2018,(11):42. [26] Lee Y W, Choi D K, Park J W. Surface chemical characterization using AES/SAM and TOF-SIMS on KOH-impregnated activated carbon by selective adsorption of NOx[J]. Industrial & Engineering Chemistry Research, 2001,40(15):3337−3345. [27] Guo Y Y, Li Y R, Zhu T Y, et al. Effects of Concentration and adsorption product on the adsorption of SO2 and NO on activated carbon[J]. Energy & Fuels, 2013,27(1):360−366. [28] Rubel A M, Stencel J M. The effect of low-concentration SO2 on the adsorption of NO from gas over activated carbon[J]. Fuel, 1997,76(6):521−526. doi: 10.1016/S0016-2361(96)00221-9 [29] Davini P. SO2 and NOx adsorption properties of activated carbons obtained from a pitch containing iron derivatives[J]. Carbon, 2001,39(14):2173−2179. doi: 10.1016/S0008-6223(01)00035-5 [30] Tang Q, Zhang Z G, Zhu W P, et al. SO2 and NO selective adsorption properties of coal-based activated carbons[J]. Fuel, 2005,84(4):461−465. doi: 10.1016/j.fuel.2004.03.010 [31] Dai Shifeng, Ren Deyi, Song Jianfang, et al. Application of XPS in research on occurrence of organic sulfur in vitrain[J]. Journal of China University of Mining, 2002,31(3):225−228. (代世峰, 任德贻, 宋建芳, 等. 应用 XPS 研究镜煤中有机硫的存在形态[J]. 中国矿业大学学报, 2002,31(3):225−228. doi: 10.3321/j.issn:1000-1964.2002.03.002 [32] Xu Ning, Tao Xiuxiang, Xie Maohua, et al. Study on variation of sulfur chemical forms in microwave desulfurized coal based on XPS[J]. Coal Engineering, 2014,46(12):111−113. (许宁, 陶秀祥, 谢茂华, 等. 基于 XPS 的微波脱硫前后煤中硫形态的变化研究[J]. 煤炭工程, 2014,46(12):111−113. doi: 10.11799/ce201412036 [33] Cai Chuanchuan, Yang Minghu, Pan Lulu, et al. Study of types and content of sulfur in coal samples of different densities using X-ray photoelectron spectroscopy(XPS)[J]. Coal Prepapation Technology, 2018,(5):56−59. (蔡川川, 杨明虎, 潘露露, 等. 基于XPS技术的不同密度煤样中硫的类型和含量研究[J]. 选煤技术, 2018,(5):56−59. [34] Liu Yifeng, Shen Benjun, Pi Zhipeng, et al. Oxidation transferring mechanism of SO2 in FCC flue gas over CeO2 surface[J]. Journal of Chemical Industry and Engineering, 2016,67(12):5015−5023. (刘逸锋, 沈本贤, 皮志鹏, 等. CeO2 表面氧化转移 FCC 烟气中 SO2 的反应过程[J]. 化工学报, 2016,67(12):5015−5023. [35] Ge Tao, Ma Xiangmei. XPS study on occurrence characteristics of carbon, oxygen, nitrogen and sulfur in coking coal[J]. Coal Technology, 2018,37(3):293−295. (葛涛, 马祥梅. 炼焦煤中碳、氧、氮、硫赋存特征的XPS研究[J]. 煤炭技术, 2018,37(3):293−295. -





 下载:
下载: