Effects of Ti element on phase stability of σ(FeV)
-
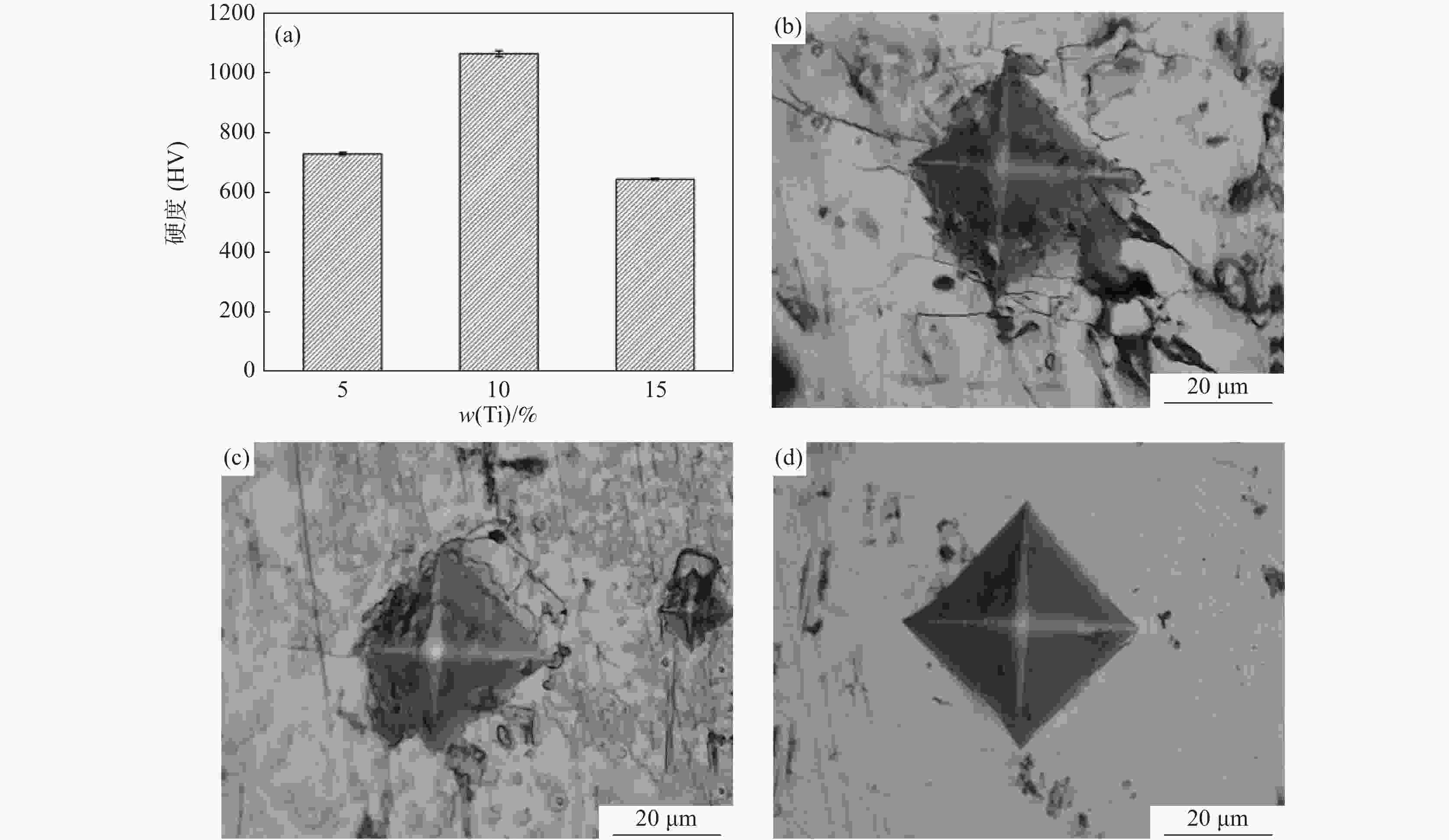
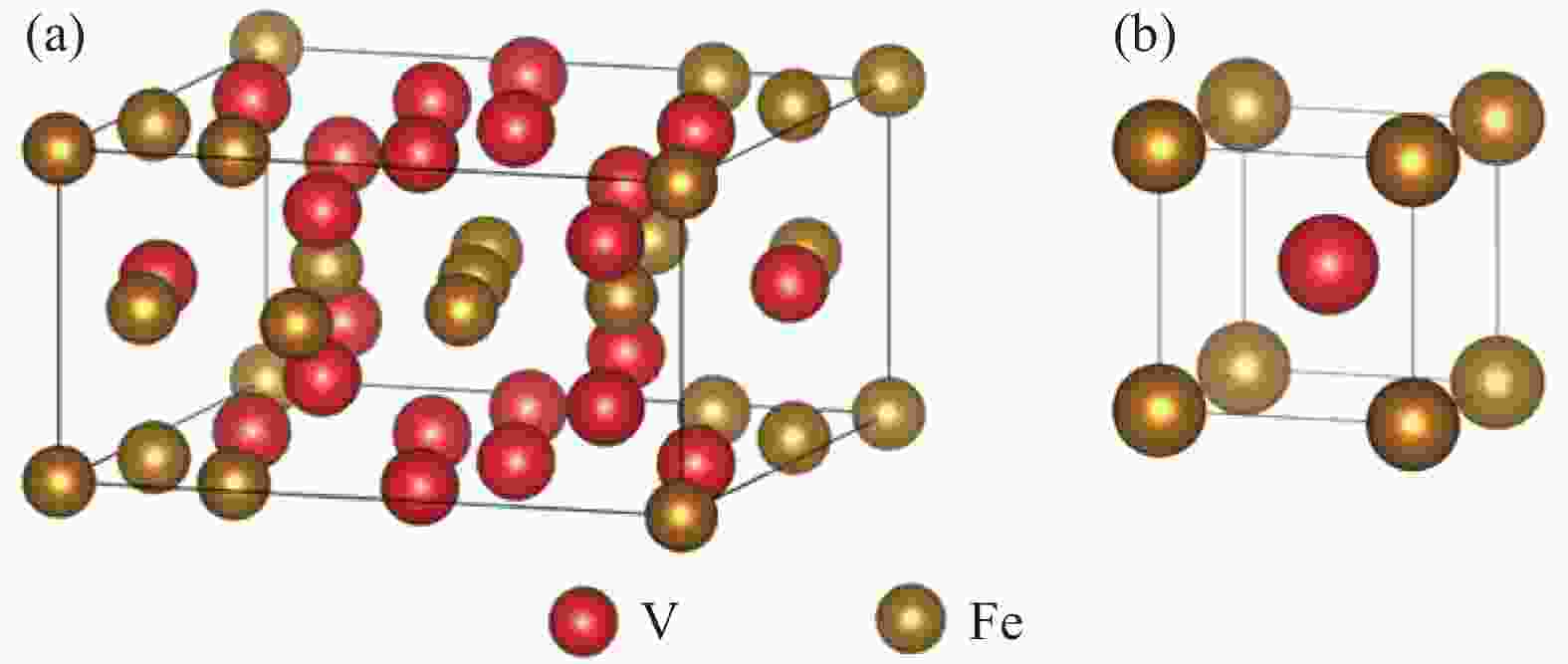
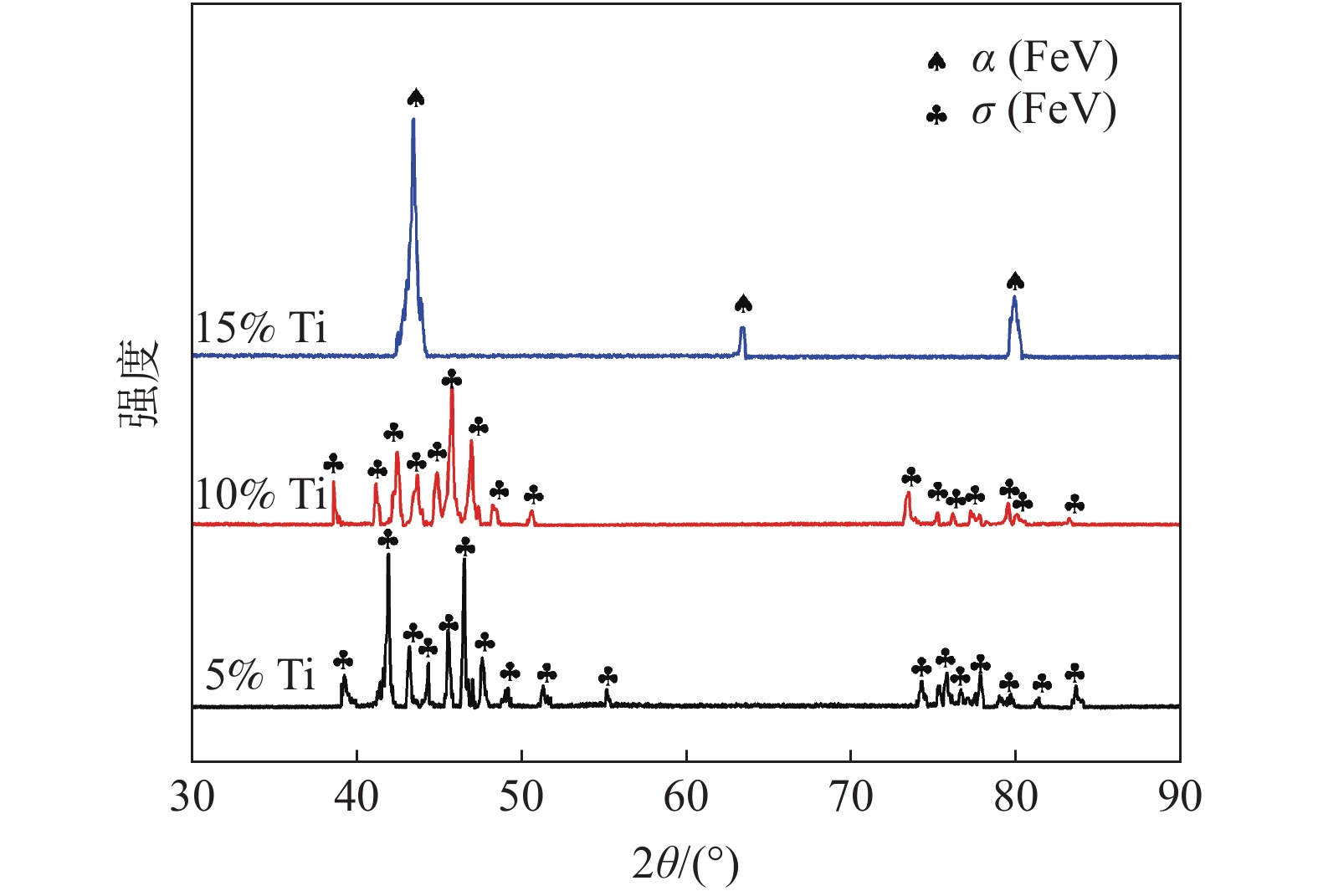
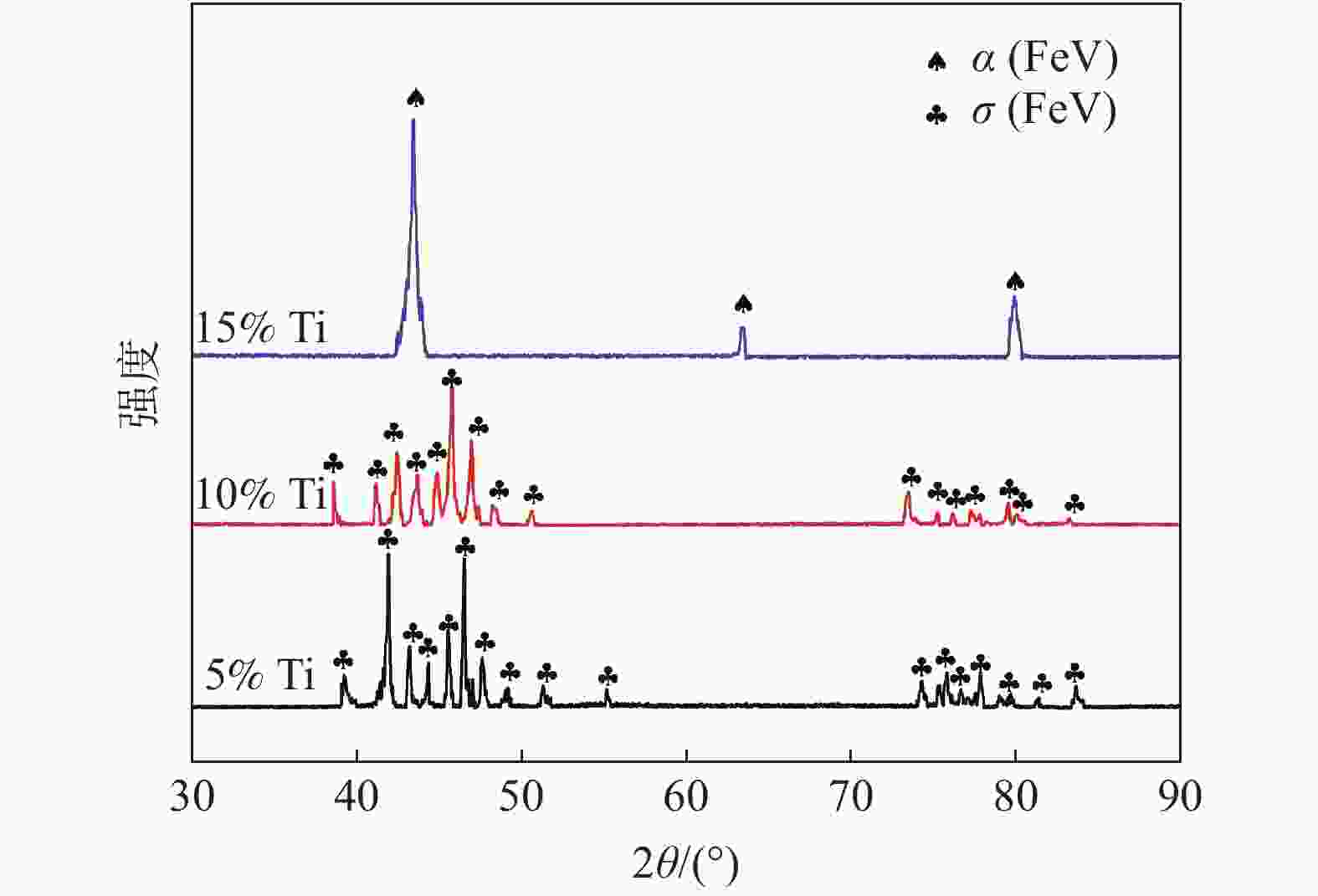
摘要: 为研究大尺寸过渡金属元素Ti对σ(FeV)物相稳定性的影响,通过电弧熔炼的方法制备了不同Ti含量的FeV合金,对其进行了物相鉴定和显微硬度分析。结果表明:随Ti含量从5%增加至15%,合金物相逐步从复杂结构的σ(FeV)物相转变为简单的体心立方α(FeV),由于Ti原子的固溶强化以及后续的σ→α相变,显微硬度呈现出先升高后降低的变化规律。Ti元素的引入降低了σ(FeV)物相的稳定性,抑制σ相的形成,原因在于随Ti含量增加,Ti元素削弱了σ相中d-d电子共价键合的主导地位,且更有利于形成配位数16的配位多面体,不满足形成σ(FeV)相的几何尺寸要求。Abstract: In order to study the effect of large-size transition-metal element Ti on σ(FeV). FeV alloys with different Ti contents were prepared by arc melting, and phase identification and microhardness testing were conducted. The results show that with the increase of Ti concentration from 5% to 15%, the phase of the alloy gradually changes from complex structure σ(FeV) into simple bcc phase α(FeV). It shows that microhardness increases first and then decreases due to the solid solution strengthening of Ti atoms and subsequent σ → α phase transition. The introduction of Ti reduces the phase stability of σ(FeV), and inhibits the formation of σ(FeV) phase. With the increase of Ti content, Ti decreases the dominating d-d electron covalent bonding in σ phase and promotes the formation of coordination polyhedron with coordination number of 16, which does not meet the geometric requirements for the formation of σ phase.
-
Key words:
- FeV /
- σ phase /
- Ti element /
- hardness /
- stability
-
表 1 钒铁的化学成分
Table 1. Chemical composition of FeV
% V C Si P S Al Mn Fe 48.03 0.04 0.07 0.04 0.01 0.11 0.06 Balance 表 2 不同拓扑密堆相的结构特征[7]
Table 2. Structural characteristics of various topologically close-packed phases
物相 单位晶胞原子数 单位晶胞不同位点的数量 CN12 CN13 CN14 CN15 CN16 Laves 12 8 4 24 16 8 μ 13 7 2 2 2 M 52 28 8 8 8 R 53 27 12 6 8 P 56 24 20 8 4 δ 56 24 20 8 4 σ 30 10 16 4 A15 8 2 6 χ 58 24 24 10 表 3 存在σ相的二元过渡金属体系的VET值[7]
Table 3. The VET of binary TM−TM systems with existence of σ phase
VET 合金体系 5+7=12 V−Mn,V−Re 5+8=13 V−Fe,Nb−Os,Ta−Os 5+9=14 V−Co,Nb−Rh,Nb−Ir,Ta−Rh,Ta−Ir 5+10=15 V−Ni,Ta−Pt 6+7=13 Cr−Mn,Cr−Tc,Cr−Re,Mo−Mn,Mo−Tc,Mo−Re,W−Tc,W−Re 6+8=14 Cr−Fe,Cr−Ru,Cr−Os,Mo−Fe,Mo−Ru,Mo−Os,W−Ru,W−Os 6+9=15 Cr−Co,Mo−Co,Mo−Ir,W−Ir 7+7=14 Mn−Tc,Mn−Re 7+8=15 Tc−Fe,Re−Fe 表 4 整数配位数以及对应的的R和1/R值[7]
Table 4. Values of R, 1/R and corresponding values of CN
CN R 1/R 11 0.884 1.131 12 0.902 1.109 13 0.976 1.025 14 0.955 1.047 15 0.896 1.116 16 0.845 1.183 17 0.801 1.248 -
[1] Frank F C, Kasper J S. Complex alloy structures regarded as sphere packings. I. Definitions basic principles[J]. Acta Crystallographica, 1958,11:184−190. doi: 10.1107/S0365110X58000487 [2] Frank F C, Kasper J S. Complex alloy structures regarded as sphere packings. II. Analysis and classification of representative structures[J]. Acta Crystallographica, 1959,12:483−499. doi: 10.1107/S0365110X59001499 [3] Joubert J M. Crystal chemistry and calphad modeling of the σ phase[J]. Progress in Materials Science, 2008,53:528−583. doi: 10.1016/j.pmatsci.2007.04.001 [4] Rae C M F, Reed R C. The precipitation of topologically close-packed phases in rhenium-containing superalloys[J]. Acta Materialia, 2001,49:4113−4125. doi: 10.1016/S1359-6454(01)00265-8 [5] Sato J, Omori T, Oikawa K, et al. Cobalt-base high-temperature alloys[J]. Science, 2006,312:90−91. doi: 10.1126/science.1121738 [6] Ustinovshchikov Y I. Sigma phase in iron-chromium alloys and steels[J]. Metal Science and Heat Treatment, 2011,53:520−525. [7] 鲜勇. Fe-V中间合金中σ相形成机理及其工业化技术应用研究[D]. 上海: 上海大学, 2016: 29-56.Xian Yong. Formation mechanism of σ phase in Fe-V alloy and its industrial application [D]. Shanghai: Shanghai University, 2016: 29-56. [8] Xian Yong. Effects of Al content on phase transformation in FeV50 alloy and its mechanism[J]. Iron Steel Vanadium Titanium, 2012,33(5):14−18. (鲜勇. Al 含量对 FeV50 合金相变的影响及机理研究[J]. 钢铁钒钛, 2012,33(5):14−18. doi: 10.7513/j.issn.1004-7638.2012.05.004 [9] Ladines A N, Hammerschmidt T, Drautz R. Structural stability of Fe-based topologically close-packed phases[J]. Intermetallics, 2015,59:59−67. doi: 10.1016/j.intermet.2014.12.009 [10] Cieślak J, Reissner M, Steiner W, et al. Magnetic moments and curie temperatures of σ-FeCr alloys[J]. Journal of Magnetism and Magnetic Materials, 2004,272-276:534−535. doi: 10.1016/j.jmmm.2003.12.001 -




 下载:
下载: