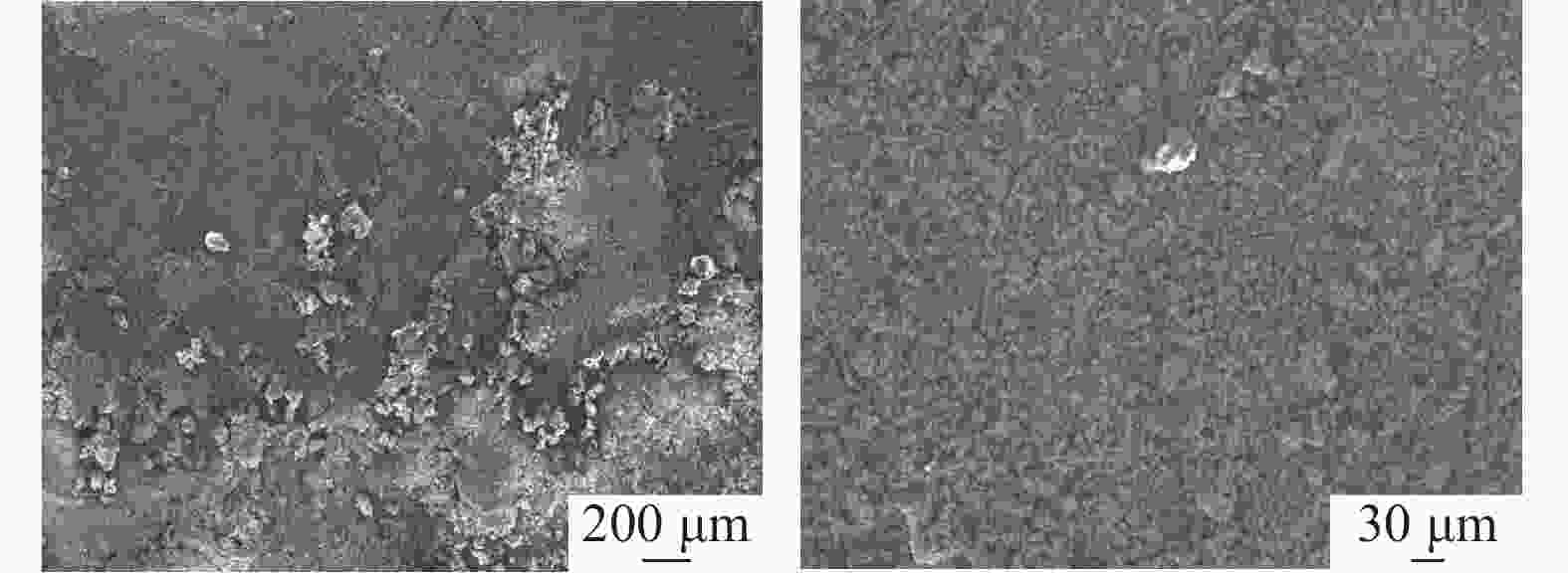
Analysis of causes of blockage of waste acid concentration heat exchanger in sulfuric acid process titanium dioxide
-
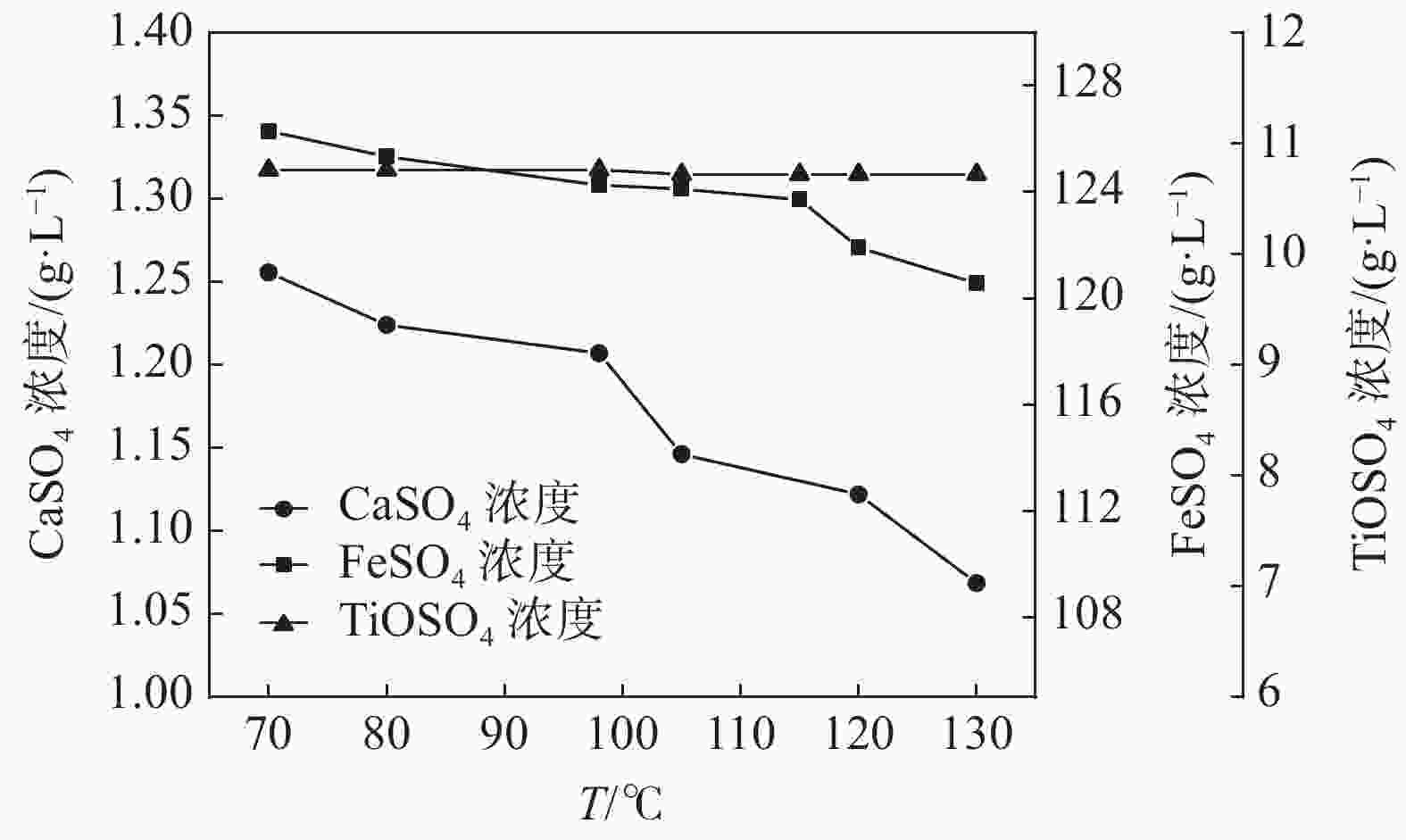
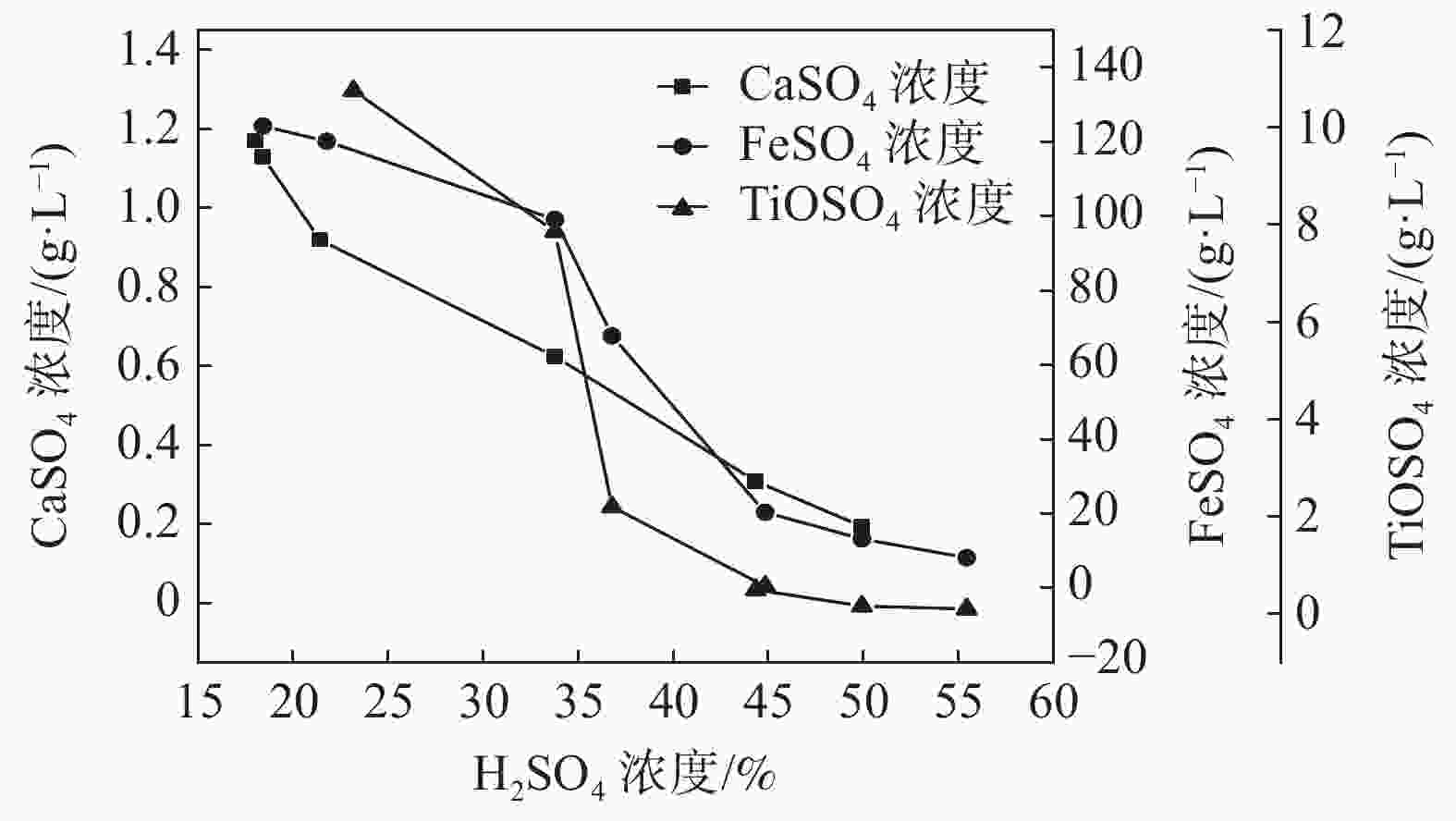
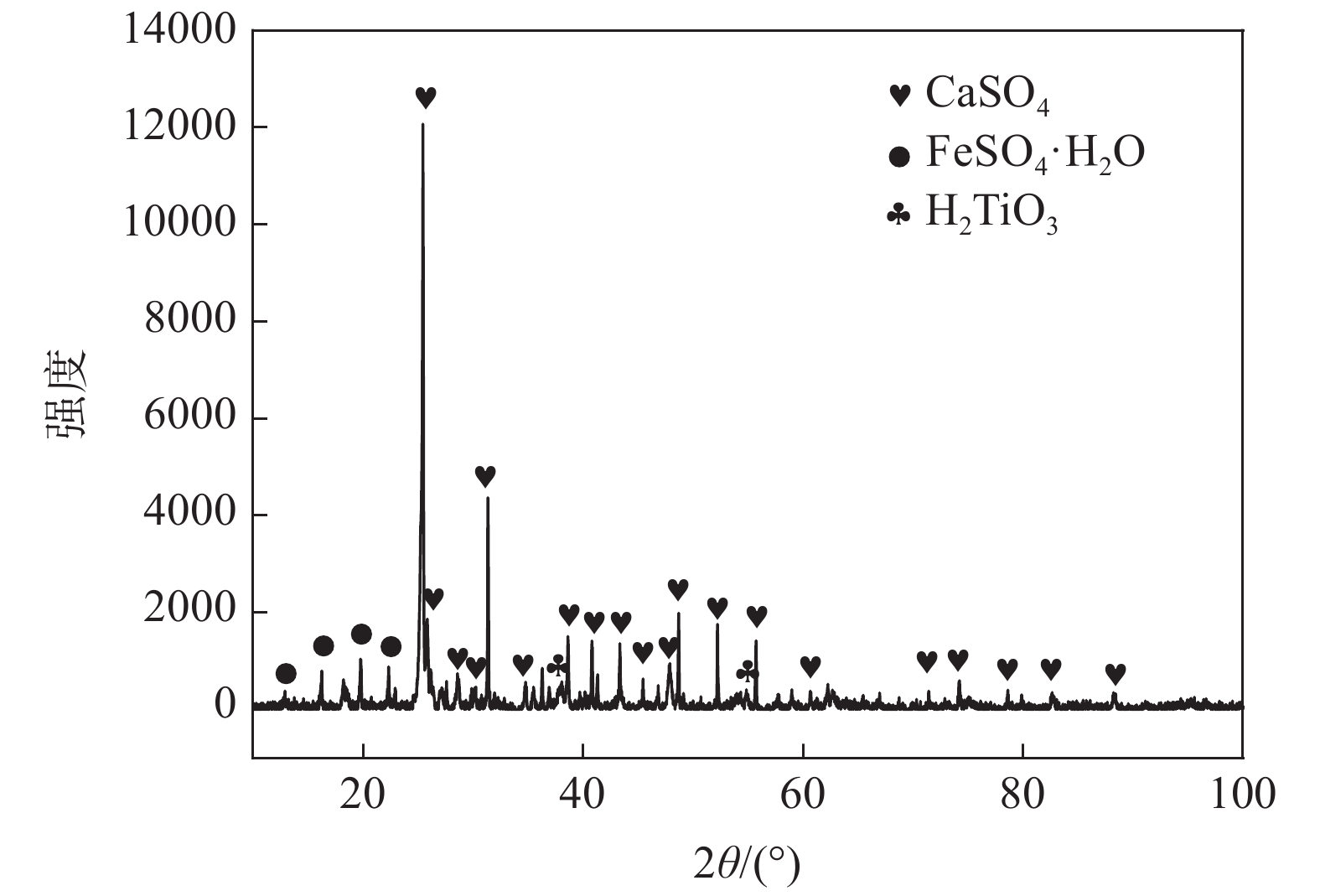
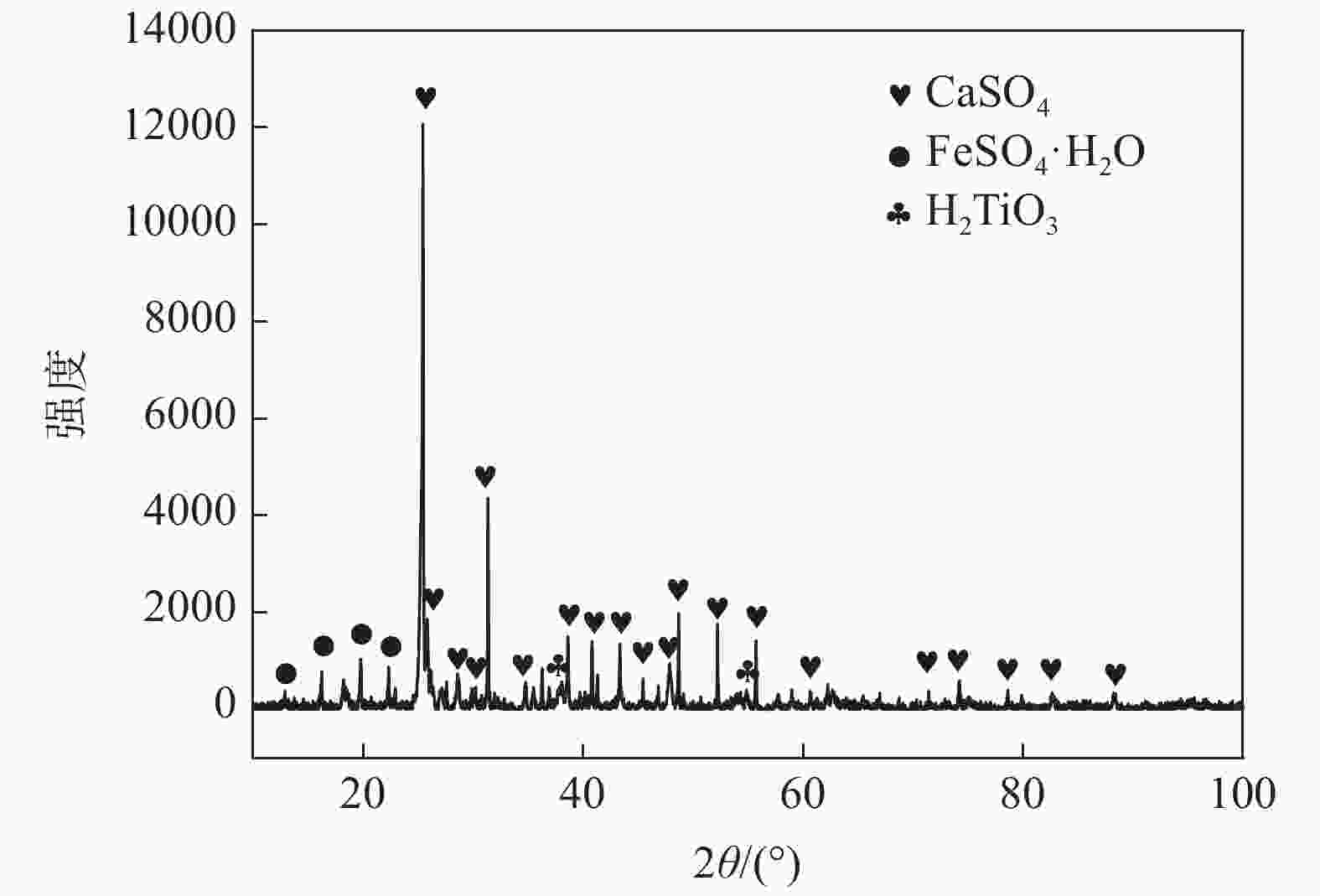
摘要: 通过化学组成分析和XRD分析,明确了硫酸法钛白废酸换热器堵塞物为以硫酸钙、一水硫酸亚铁及偏钛酸为主的混合物,三者分别来源于20废酸中硫酸钙、硫酸亚铁及硫酸氧钛。在实验室研究了废酸温度及浓度对硫酸钙、硫酸亚铁及硫酸氧钛浓度的影响,研究结果表明:在废酸温度为107 ℃,废酸浓度由18.41%提升至49.93%时,硫酸钙、硫酸亚铁及硫酸氧钛的浓度分别由1.26、126.29、10.76 g/L降低至0.19、13.23、0.16 g/L。现场浓缩废酸各级的硫酸钙、硫酸亚铁及硫酸氧钛浓度变化规律及数据与实验室研究规律及数据基本一致。研究结果为后续解决换热器堵塞提供了重要的数据及理论支撑。Abstract: The blockage of the waste acid heat exchanger of sulfuric acid process titanium dioxide had been investigated through chemical composition analysis and XRD analysis, and caused by a mixture of calcium sulfate, ferrous sulfate monohydrate and meta-titanic acid. These mixture is derived from calcium sulfate, ferrous sulfate and titanyl sulfate in 20% waste acids. The influence of waste acid temperature and concentration on the concentration of calcium sulfate, ferrous sulfate and titanyl sulfate was studied in the laboratory. The results showed that when the waste acid temperature was 107 ℃ and the waste acid concentration increased from 18.41% to 49.93%, the concentration of calcium sulfate, ferrous sulfate and titanyl sulfate decreased from 1.26 g/L, 126.29 g/L and 10.76 g/L to 0.19 g/L, 13.23 g/L and 0.16 g/L, respectively. The concentration changing rule and data of calcium sulfate, ferrous sulfate and titanium oxysulfate in concentrated waste acid at all levels are basically consistent with those of laboratory research. The research results provide important data and theoretical support for the solution of heat exchanger blockage.
-
Key words:
- sulfate process titanium dioxide /
- waste acid /
- concentration /
- heat exchanger /
- blockage
-
表 1 20废酸主要化学成分
Table 1. Main chemical components of 20 waste acid
g/L H2SO4 CaSO4 FeSO4 TiOSO4 MgSO4 MnSO4 Al2(SO4)3 H2SiO3 245.00 1.26 126.29 10.76 6.36 0.64 9.99 1.64 表 2 换热器堵塞物主要化学成分
Table 2. Main chemical components of heat exchanger blockage
% CaSO4 FeSO4 MnSO4 SiO2 H2TiO3 Al2(SO4)3 MgSO4 37.57 33.07 1.14 1.36 24.15 0.39 2.24 表 3 现场废酸浓缩过程废酸成分变化
Table 3. Compositions change of waste acid during field concentration
样品 编号 温度/℃ H2SO4/% 废酸成分/(g·L−1) CaSO4 FeSO4 TiOSO4 20废酸 0-1 ~40 23.18 1.23 124.86 10.40 0-2 19.68 1.28 120.19 10.78 均值 21.43 1.26 122.52 10.59 一级浓缩酸 1-1 107 36.78 0.37 67.86 2.20 1-2 37.00 0.31 62.43 1.94 均值 36.89 0.34 65.14 2.07 二级浓缩酸 2-1 85 55.44 0.20 8.14 0.64 2-2 75 44.41 0.17 18.32 0.34 均值 80 49.93 0.19 13.24 0.50 -
[1] Karimia L, Yazdanshenas M E, Khajavi R, et al. Optimizing the photocatalytic properties and the synergistic effects of graphene and nano titanium dioxide immobilized on cotton fabric[J]. Applied Surface Science, 2015,332:665−673. doi: 10.1016/j.apsusc.2015.01.184 [2] Romanovska N I, Manoryk P A, Ermokhina N I, et al. Effect of structural and dimensional characteristics of TiO2 and its photocatalytic activity in the oxidation of tetracycline[J]. Theoretical and Experimental Chemistry, 2019,55(5):345−353. doi: 10.1007/s11237-019-09627-0 [3] Sobczyk-guzenda Anna, Szymanski Witold, Jedrzejczak Anna, et al. Bactericidal and photowetting effects of titanium dioxide coatings doped with iron and copper/fluorine deposited on stainless steel substrates[J]. Surface & Coatings Technology, 2018,347:66−75. [4] Matsukura A, Onoda H. Influences of additives on phosphoric acid treatment of titanium dioxide as a novel white pigment[J]. Journal of Advanced Ceramics, 2015,4(3):211−216. doi: 10.1007/s40145-015-0151-3 [5] Kang J, Okabe T H. Removal of iron from titanium ore by selective chlorination using TiCl4 under oxygen content atmosphere[J]. International Journal of Mineral Processing, 2016,149:111−118. [6] Bi Sheng. Basic situation and development prospect of titanium dioxide industry in China in recent years[J]. Iron Steel Vanadium Titanium, 2021,42(2):1−4. (毕胜. 近年中国钛白粉行业基本状况及发展展望[J]. 钢铁钒钛, 2021,42(2):1−4. [7] Zhu Xiaobo, Wang Tao, Li Wang. Experimental study and kinetic analysis of red mud dealkalization from titanium dioxide waste acid leaching[J]. Bulletin of the Chinese Ceramic Society, 2020,39(12):3918−3923. (朱晓波, 王涛, 李望. 钛白废酸浸出赤泥脱碱试验研究与动力学分析[J]. 硅酸盐通报, 2020,39(12):3918−3923. [8] Li Jun, Wu Enhui, Hou Jing, et al. Experimental study on direct acid leaching of vanadium-bearing steel slag with new sulfuric acid and titanium dioxide waste acid[J]. Iron Steel Vanadium Titanium, 2020,41(3):16−22. (李军, 吴恩辉, 侯静, 等. 利用新硫酸和钛白废酸直接酸浸含钒钢渣试验研究[J]. 钢铁钒钛, 2020,41(3):16−22. doi: 10.7513/j.issn.1004-7638.2020.03.002 [9] 杨海舟, 秦玲玲, 陈钢. 钛白废酸回收及综合利用研究进展[J]. 广东化工, 2018, 45(16): 118-119.Yang Haizhou, Qin Lingling, Chen Gang. Research progress on recovery and comprehensive utilization of titanium dioxide waste acid [J]. Guangdong Chemical Industry, 2018, 45 (16): 118-119. [10] Wang Heqing, Le Qinghua, Xu Jumei, et al. Study on the removal of ferrous sulfate from titanium dioxide waste acid by solution crystallization[J]. Journal of Chemical Engineering, 2015,29(3):697−702. (王和庆, 乐清华, 徐菊美, 等. 溶析结晶法脱除钛白废酸中硫酸亚铁盐的研究[J]. 高校化学工程学报, 2015,29(3):697−702. [11] 李志云. 硫酸法钛白工艺换热器的清洗、阻垢研究[D]. 重庆: 重庆大学, 2011.Li Zhiyun. Study on cleaning and scale inhibition of titanium dioxide heat exchanger by sulfuric acid process [D]. Chongqing: Chongqing University, 2011. [12] Wang Haibo, Wang Bin. Research on decalcification of metatitanate[J]. Iron Steel Vanadium Titanium, 2015,36(6):18−22. (王海波, 王斌. 偏钛酸脱钙研究[J]. 钢铁钒钛, 2015,36(6):18−22. doi: 10.7513/j.issn.1004-7638.2015.06.004 [13] Zhu Jiabing, Zhong Hui, Liu Shandong, et al. The solubility of calcium sulfate in high temperature salt solution[J]. Technology and Development of Chemical Industry, 2015,44(12):13−14. (朱佳兵, 钟辉, 刘善东, 等. 硫酸钙在高温盐溶液中的溶解度[J]. 化工技术与开发, 2015,44(12):13−14. doi: 10.3969/j.issn.1671-9905.2015.12.005 [14] He Wei, Wu Xiaoqin, Liu Fang. Study on the solubility of calcium sulfate in the conversion process of Ca-Mg-K-Cl-H2O system[J]. Environmental Science and Technology, 2010,(5):35−38. (何伟, 吴晓琴, 刘芳. 硫酸钙在Ca-Mg-K-Cl-H2O体系转化过程中溶解度研究[J]. 环境科学与技术, 2010,(5):35−38. doi: 10.3969/j.issn.1003-6504.2010.05.008 [15] Mu Ping, Yang Zhengyu, Zhao Liang, et al. Non-isothermal kinetics of thermal decomposition and dehydration of ferrous sulfate heptahydrate[J]. Journal of Hebei Normal University, 1997,4(2):55−58. (穆平, 杨正宇, 赵良, 等. 七水合硫酸亚铁热分解及脱水非等温动力学研究[J]. 河北师范大学学报, 1997,4(2):55−58. -




 下载:
下载: