Experimental study on decarbonization of Fe -C-Mn thin strips in Ar-H2O-H2 atmosphere
-
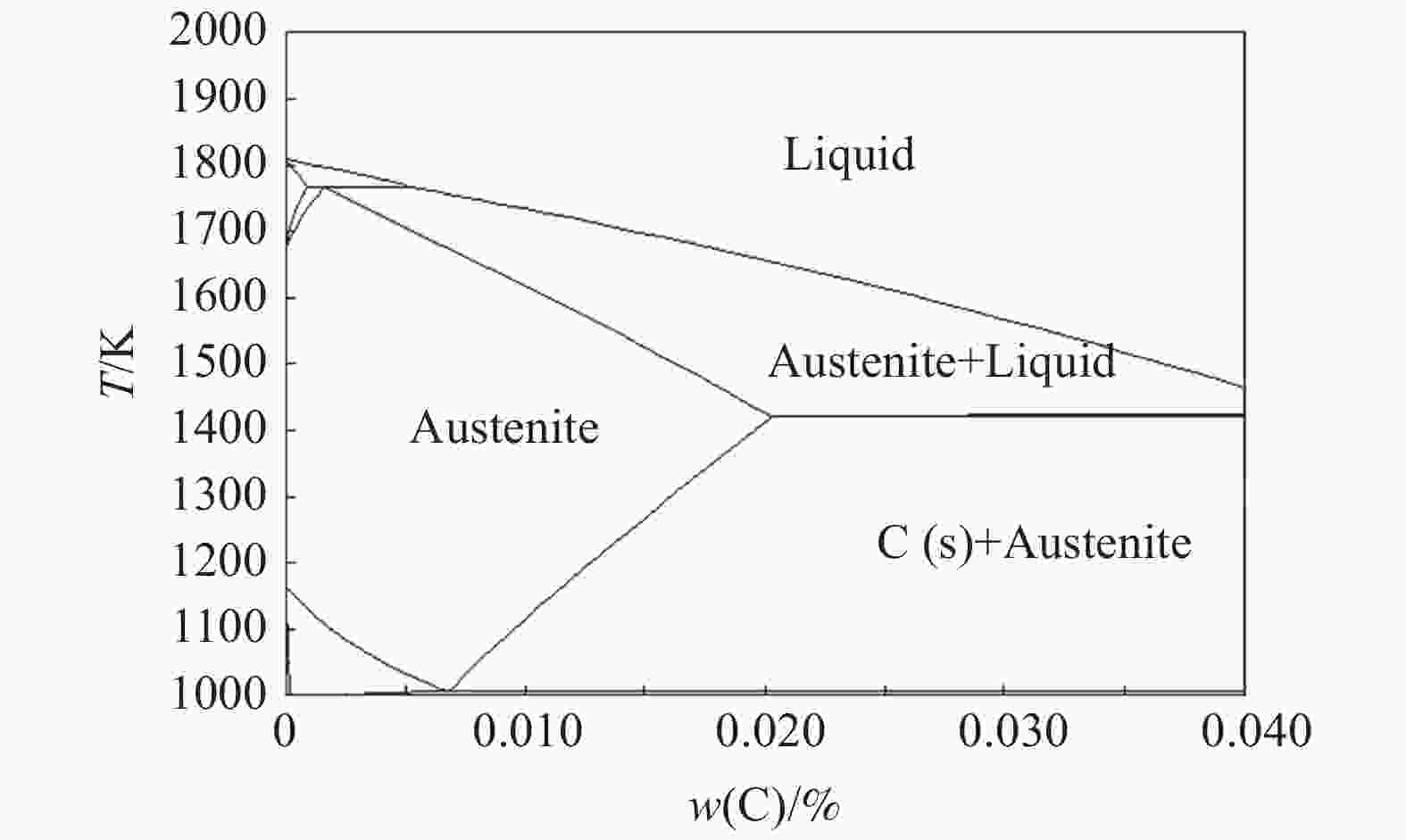
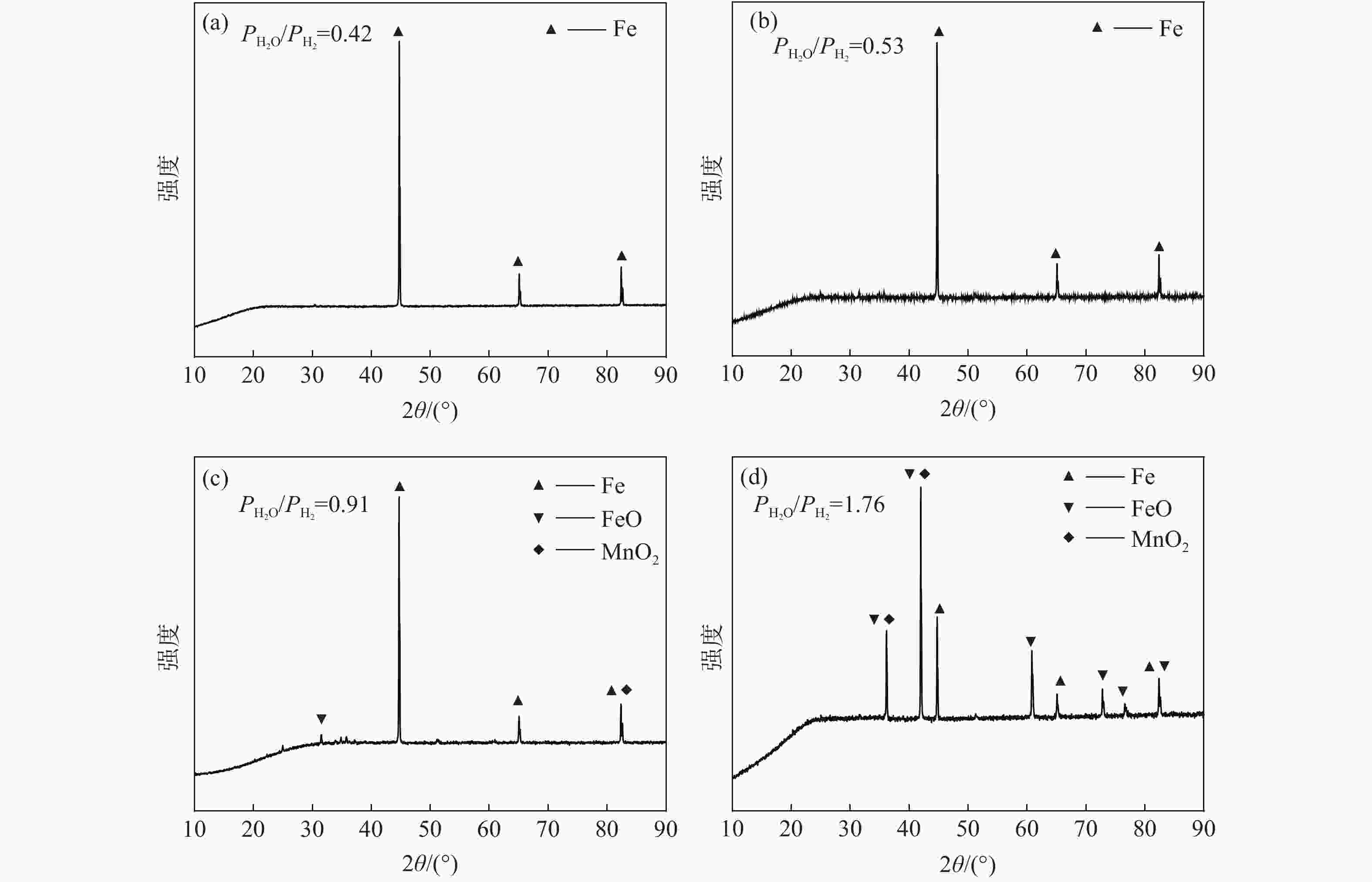
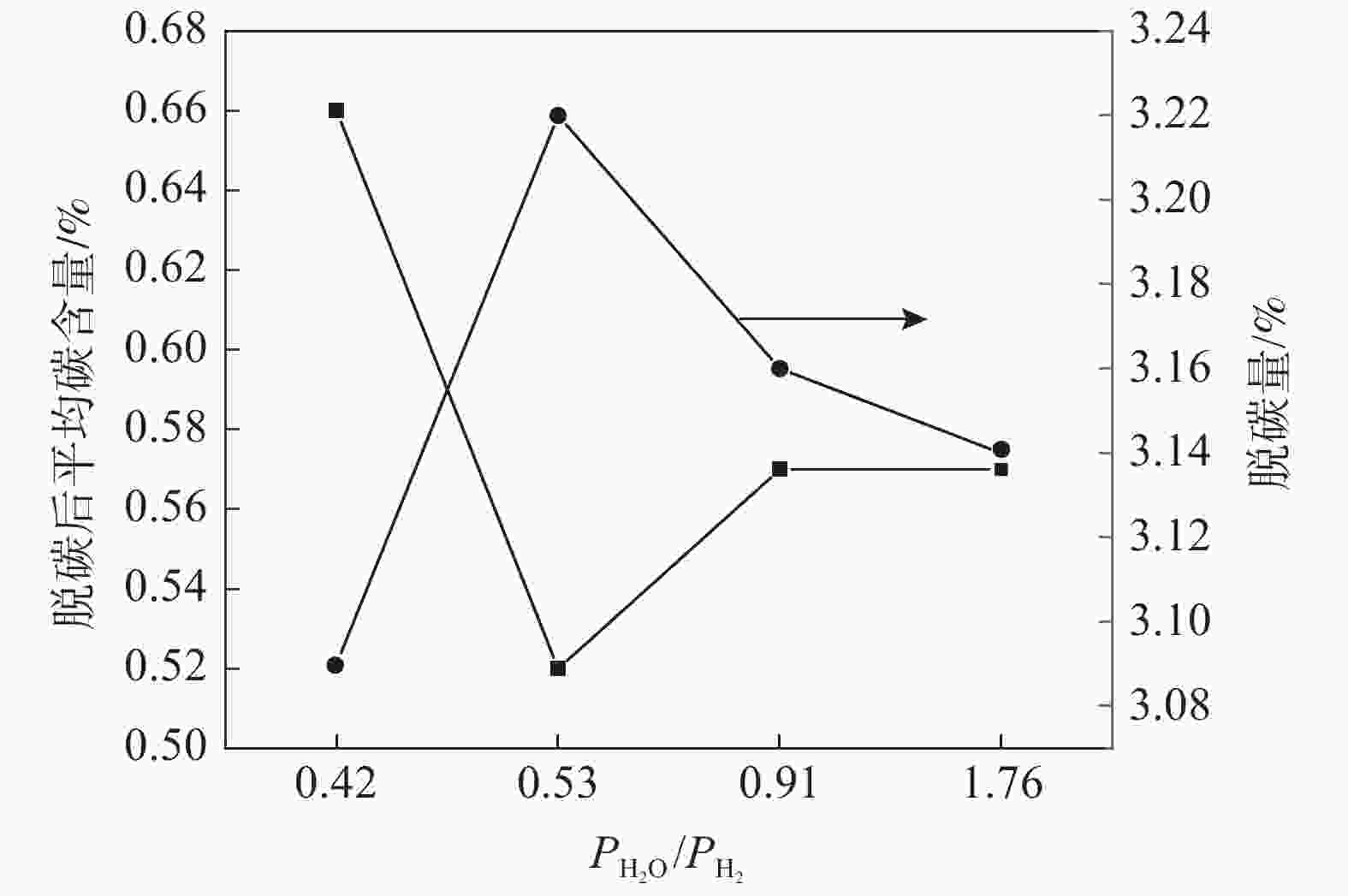
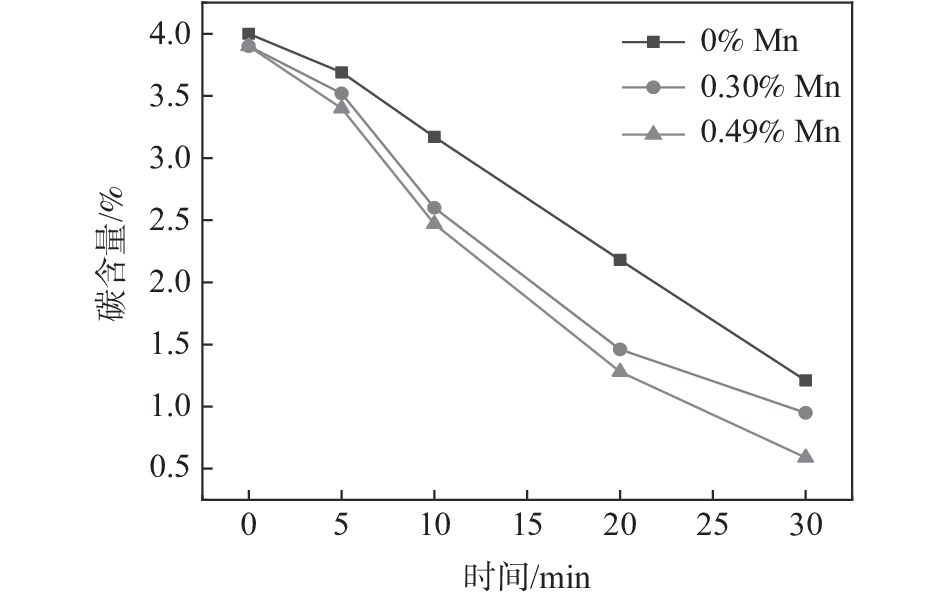
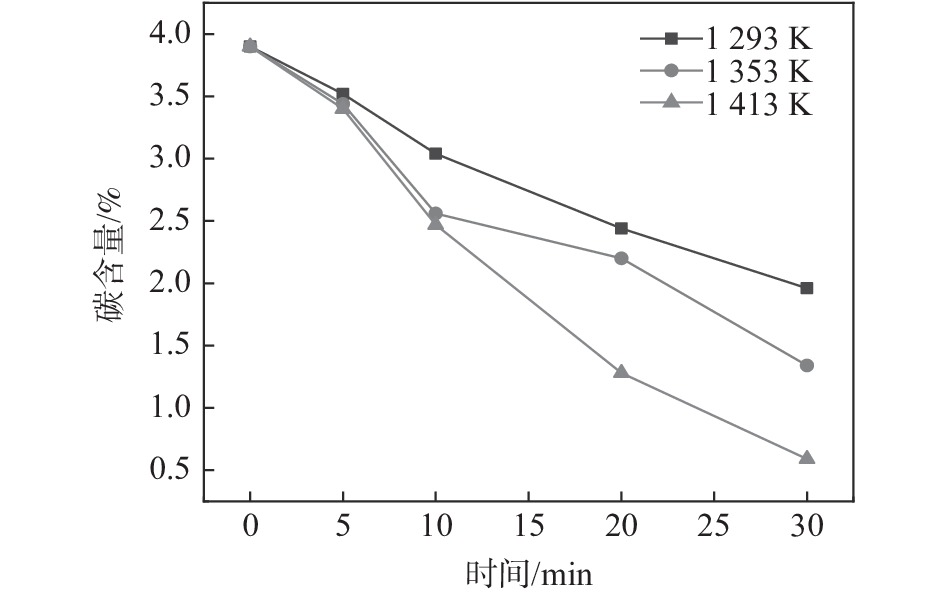
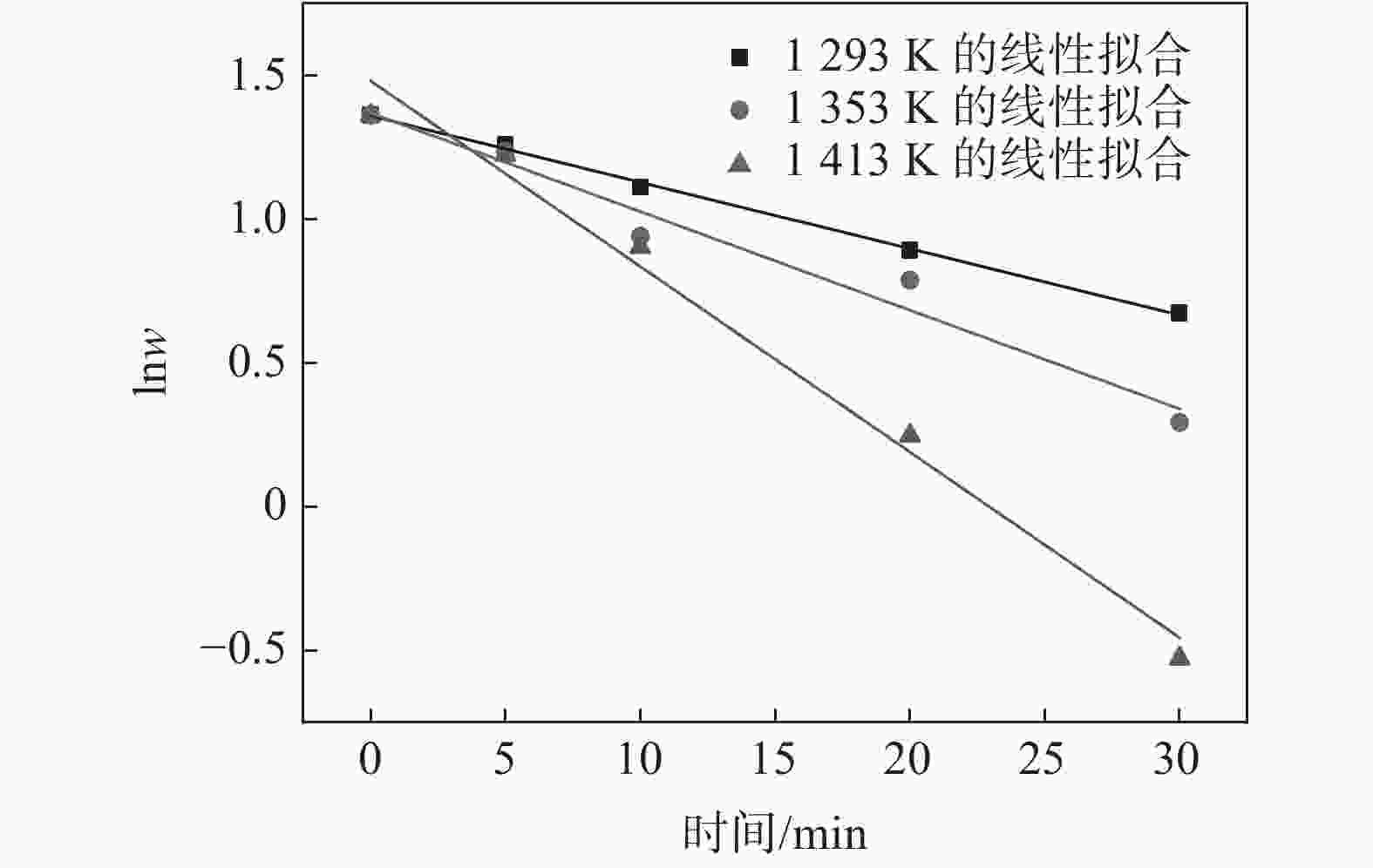
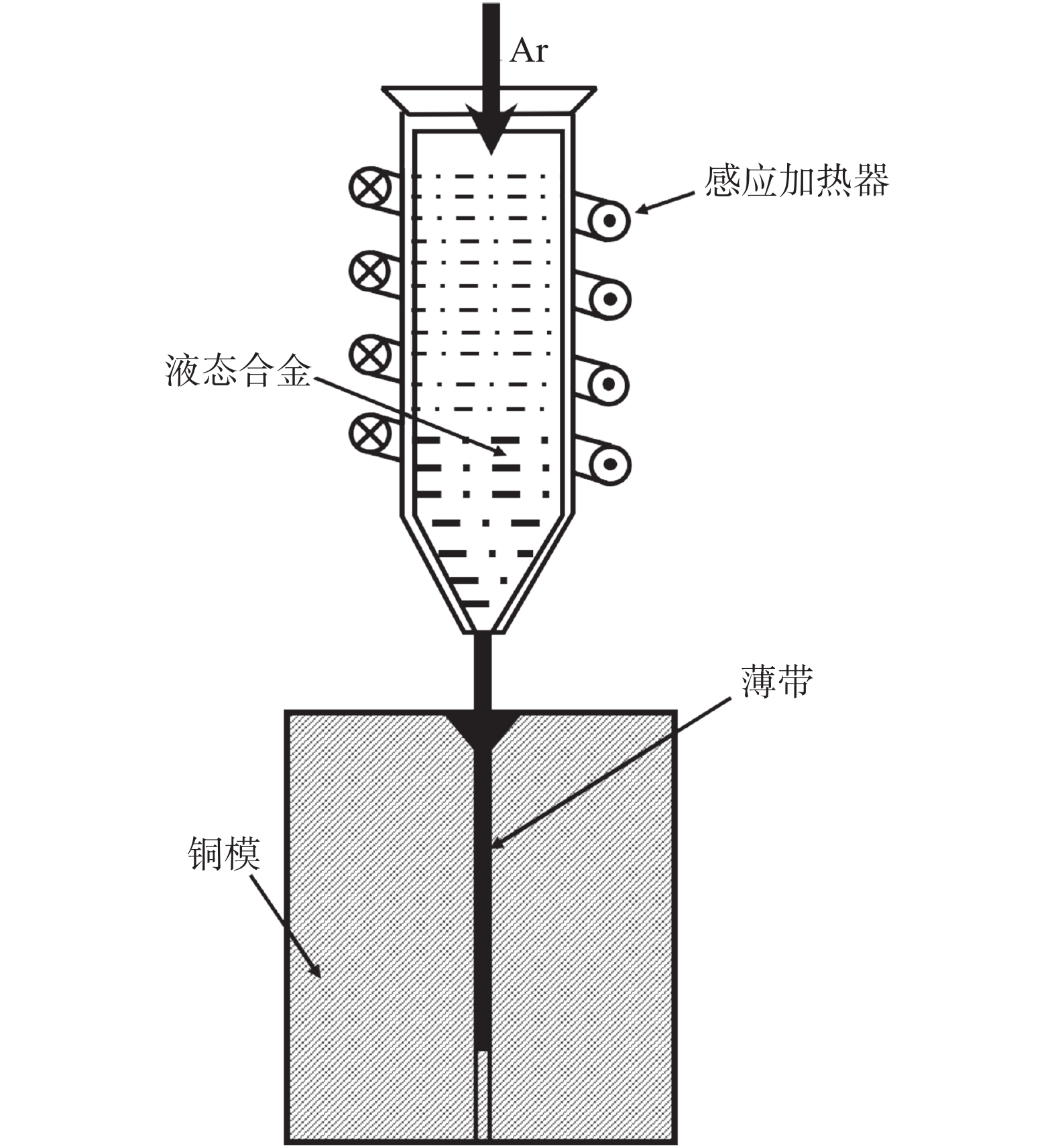
摘要: 为研究Ar-H2O-H2气氛下含锰铁碳合金薄带不同锰含量、脱碳温度对脱碳效果的影响,以初始碳含量为3.90%,锰含量分别为0、0.30%、0.49 %的1 mm厚的含锰铁碳合金薄带为研究对象,在Ar-H2O-H2的弱氧化性气氛下进行脱碳试验,通过对不同气氛条件下薄带表面进行XRD检测分析,表明脱碳气氛PH2O/PH2不宜超过0.53。分别在1293、1353、1413 K温度下脱碳5、10、20、30 min。结果表明:在一定范围内随合金薄带中锰含量的增加,平均脱碳量增加;脱碳温度的升高显著提高了薄带的平均脱碳量。Abstract: In order to study the influence of Mn content and decarburization temperature on decarburization effect of ferromanganese carbon alloy thin strip in Ar-H2O-H2 atmosphere, the ferromanganese carbon alloy thin strip in 1mm thickness with initial carbon content of 3.90% and manganese content of 0, 0.30% and 0.49% was used to conduct decarburization experiment in the weak oxidizing atmosphere of Ar-H2O-H2. XRD analysis was done on the surface of thin strip after decarburization under different atmosphere conditions. It is indicated that the decarbonization atmosphere PH2O/PH2 should not exceed 0.53. Decarburization was carried out at 1 293, 1 353 and 1 413 K for 5, 10, 20 and 30 min, respectively. The results show that the amount of decarburization increases obviously with increasing Mn content. The increase of decarburization temperature significantly increases average decarburization amount of thin strip.
-
Key words:
- solid-state steelmaking /
- Fe-C-Mn alloy /
- thin strip /
- gas-solid reaction /
- decarburization
-
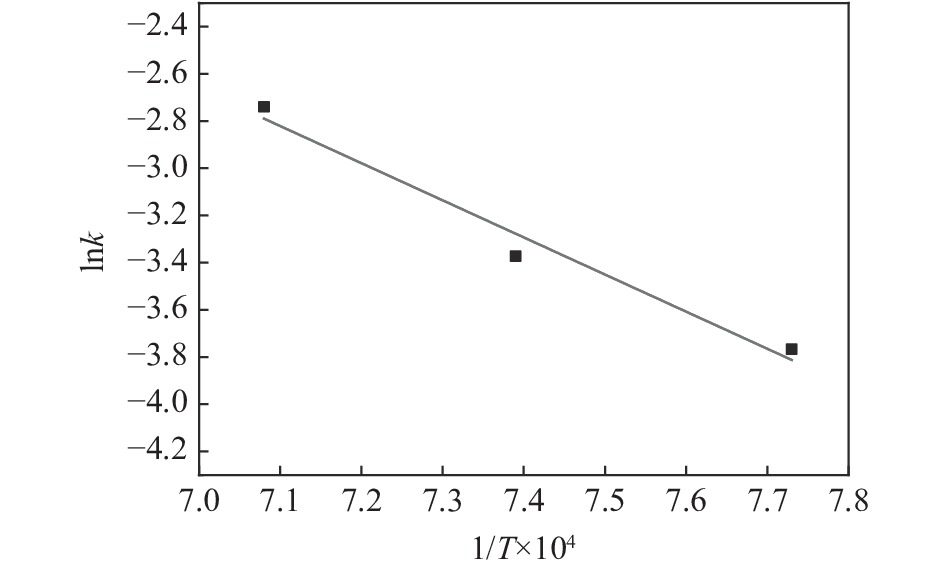
表 1 各温度下的(1/T)×104和lnk
Table 1. (1/T)×104 and lnk at different temperature
温度/K (1/T)×104/K−1 k/min−1 lnk 1293 7.73 0.02312 −3.76706 1353 7.39 0.0343 −3.37261 1413 7.08 0.06456 −2.74016 -
[1] Li Bing, Li Xinchuang, Li Chuang. The latest progress in the efficient use of energy in the steel industry at home and abroad[J]. Engineering Studies - Engineering with an Interdisciplinary Perspective, 2017,9(1):68−77. (李冰, 李新创, 李闯. 国内外钢铁工业能源高效利用最新进展[J]. 工程研究-跨学科视野中的工程, 2017,9(1):68−77. [2] Park J O, Long T V, Sasaki Y. Feasibility of solid-state steelmaking from cast iron-decarburization of rapidly solidified cast iron[J]. The Iron and Steel Institute of Japan, 2012,52(1):26−34. doi: 10.2355/isijinternational.52.26 [3] Mcdonald. Solid state steelmaking: process technical and economic viability[J]. Ironmaking & Steelmaking, 2012,39(7):487−489. [4] Ebrahim, Mostafa, Jalil. A new approach in solid state steelmaking from thin cast iron sheets through decarburization in CaCO3 pack[J]. ISIJ Internation,2018, 58(10): 1791-1800. [5] Chen Ting, Xu Guang. Research on component gradient steel materials[J]. Hot Working Technology, 2011,40(6):47−50. (陈婷, 徐光. 成分梯度钢铁材料的研究[J]. 热加工工艺, 2011,40(6):47−50. doi: 10.3969/j.issn.1001-3814.2011.06.014 [6] Hou Yaobin, Ai Liqun, Hong Lukuo, et al. Decarbonization of Fe-C alloy by gas solid reaction in CO /CO2 atmosphere[J]. Iron and Steel, 2020,55(11):133−139. (侯耀斌, 艾立群, 洪陆阔, 等. CO/CO2气氛下Fe-C合金气固反应脱碳[J]. 钢铁, 2020,55(11):133−139. [7] Li Yaqiang, Ai Liqun, Li Qiang, et al. Decarburization test of 1 mm iron-carbon alloy thin strip gas solid reaction[J]. Iron and Steel, 2017,52(5):19−23,35. (李亚强, 艾立群, 李强, 等. 1 mm铁碳合金薄带气-固反应脱碳试验[J]. 钢铁, 2017,52(5):19−23,35. [8] 周美洁, 艾立群, 洪陆阔, 等. CO2和H2O气氛下Fe-C合金薄带脱碳对比[J/OL]. 钢铁: 1-10[2021-04-01].Zhou Meijie, Ai Liqun, Hong Lukuo, et al. Decarbonization of thin strips of Fe-C alloy in CO2 and H2O atmosphere[J/OL]. Iron and steel: 1-10[2021-04-01]. [9] 艾立群, 侯耀斌, 洪陆阔, 等. H2/H2O气氛下Fe-C合金薄带气固脱碳反应动力学[J/OL]. 工程科学学报: 1-12[2021-04-01].Ai Liqun, Hou Yaobin, Hong Lukuo. Kinetics of gas solid decarbonization of thin strip Fe-C alloy in H2/H2O atmosphere[J/OL]. Journal of Engineering Sciences: 1-12[2021-04-01] [10] 雍岐龙. 钢铁材料中的第二相[M]. 北京: 冶金工业出版社, 2006.Yong Qilong. Second phase of steel materials[M]. Beijing: Metallurgical Industry Press, 2006. [11] 宋维锡. 金属学[M]. 北京: 冶金工业出版社, 1980.Song Weixi. Metallurgy[M]. Beijing: Metallurgical Industry Press, 1980. [12] Nutting J. Engineering physical metallurgy and heat-treatment[J]. Acta Crystallographica Section B, 1979,36(10):2509−2510. [13] Callister W D, Rethwisch D G. Material science and engineering: an introduction[J]. Research and Exploration in Laboratory, 2007,7(3):227. [14] Yamada S, Konno T, Goto S, et al. Some behaviors and characteristics of decarburized layer in spheroidal graphite cast iron[J]. International Journal of the Society of Materials Eengineering for Resources, 2002,10(1):88. doi: 10.5188/ijsmer.10.88 -





 下载:
下载: