Transformation of the spine in Mg-treated non-quenched and tempered steel
-
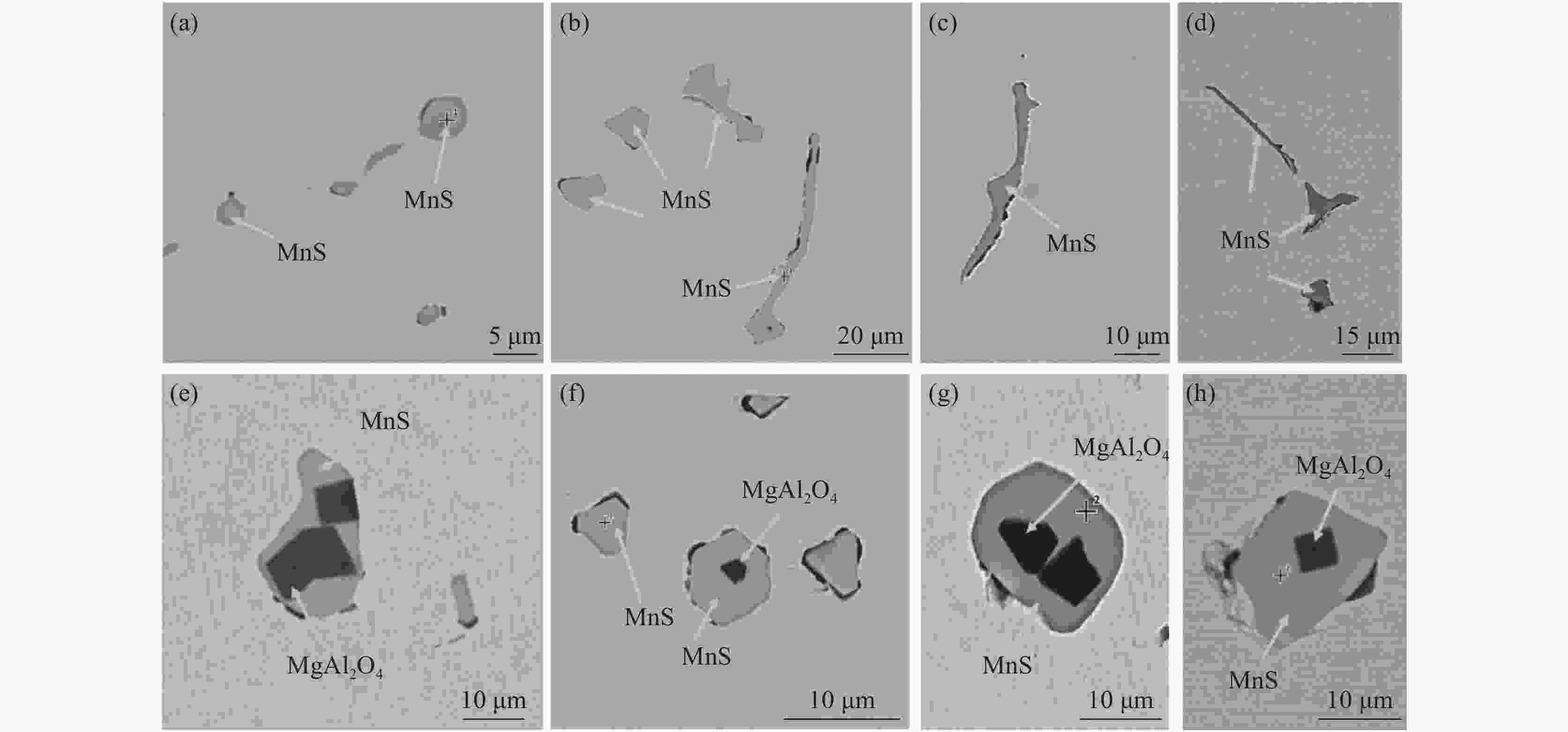
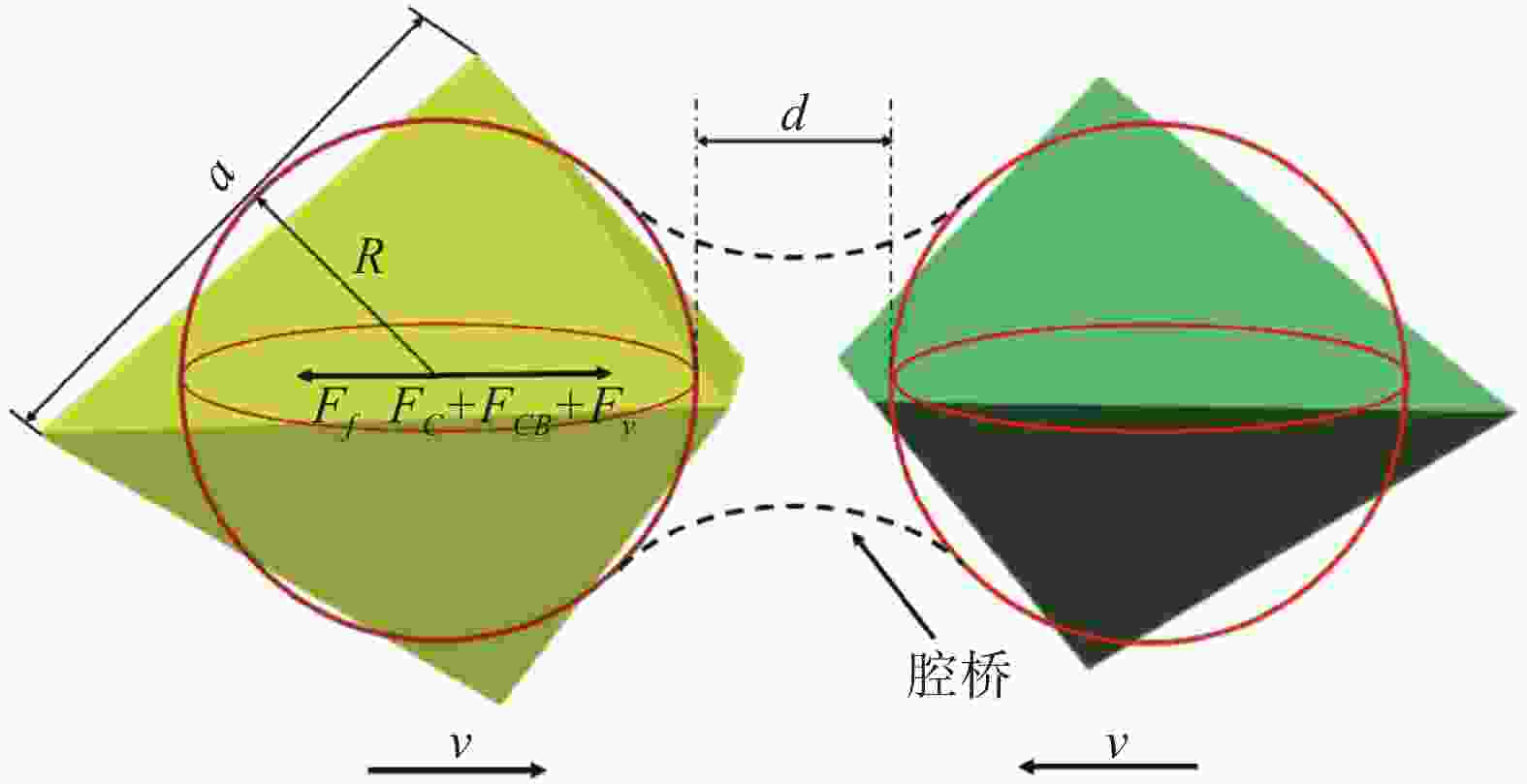
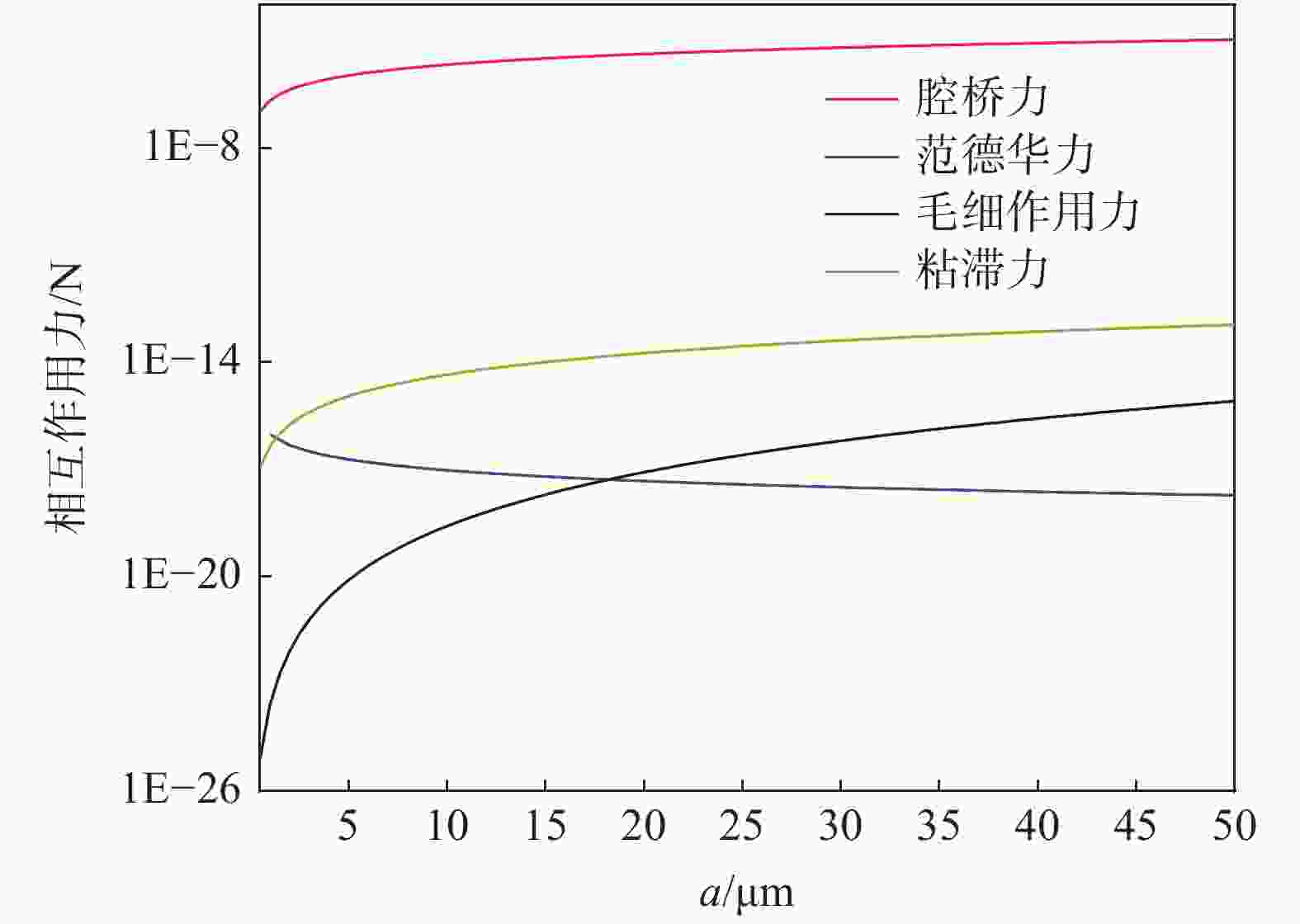
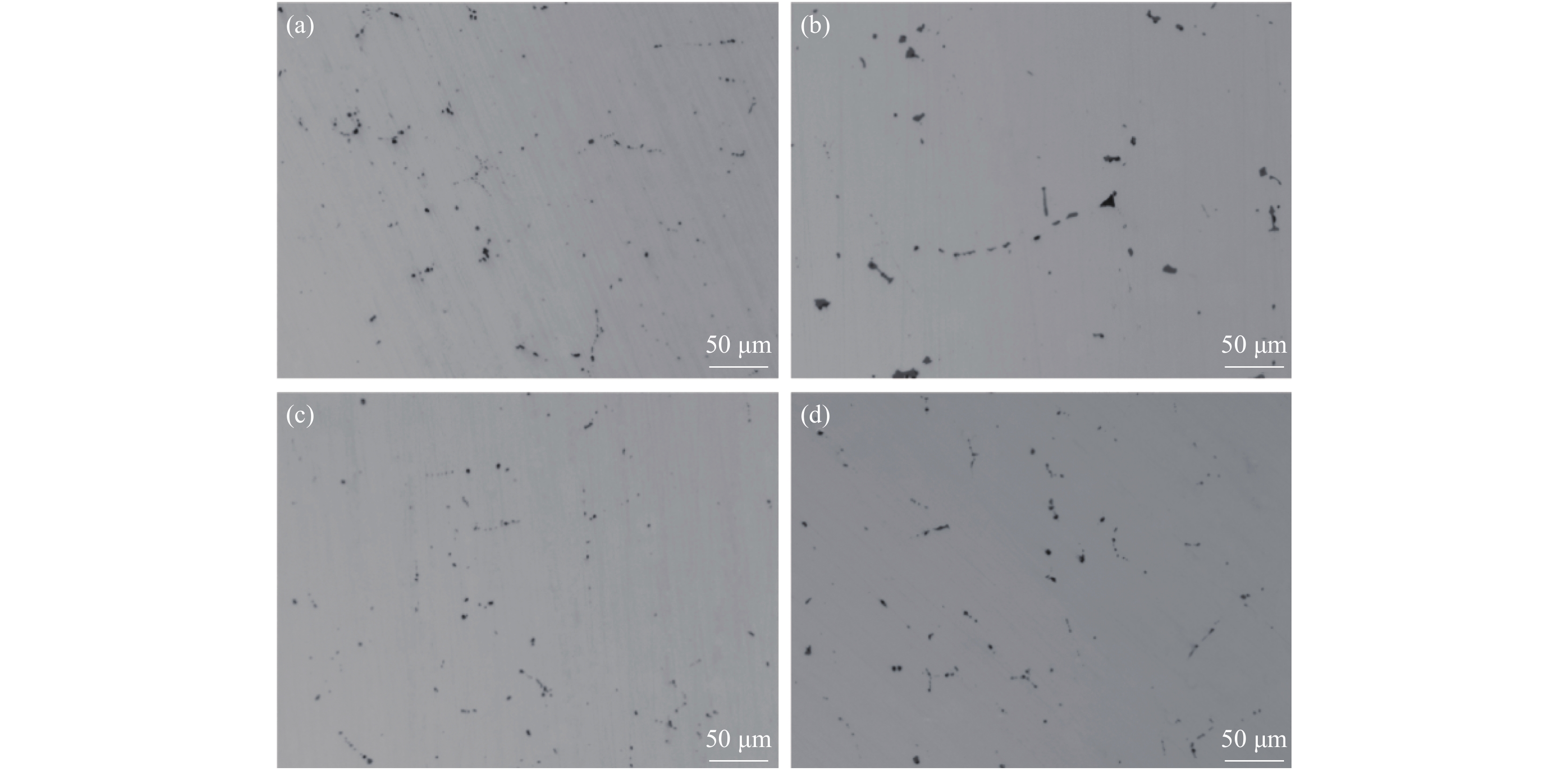
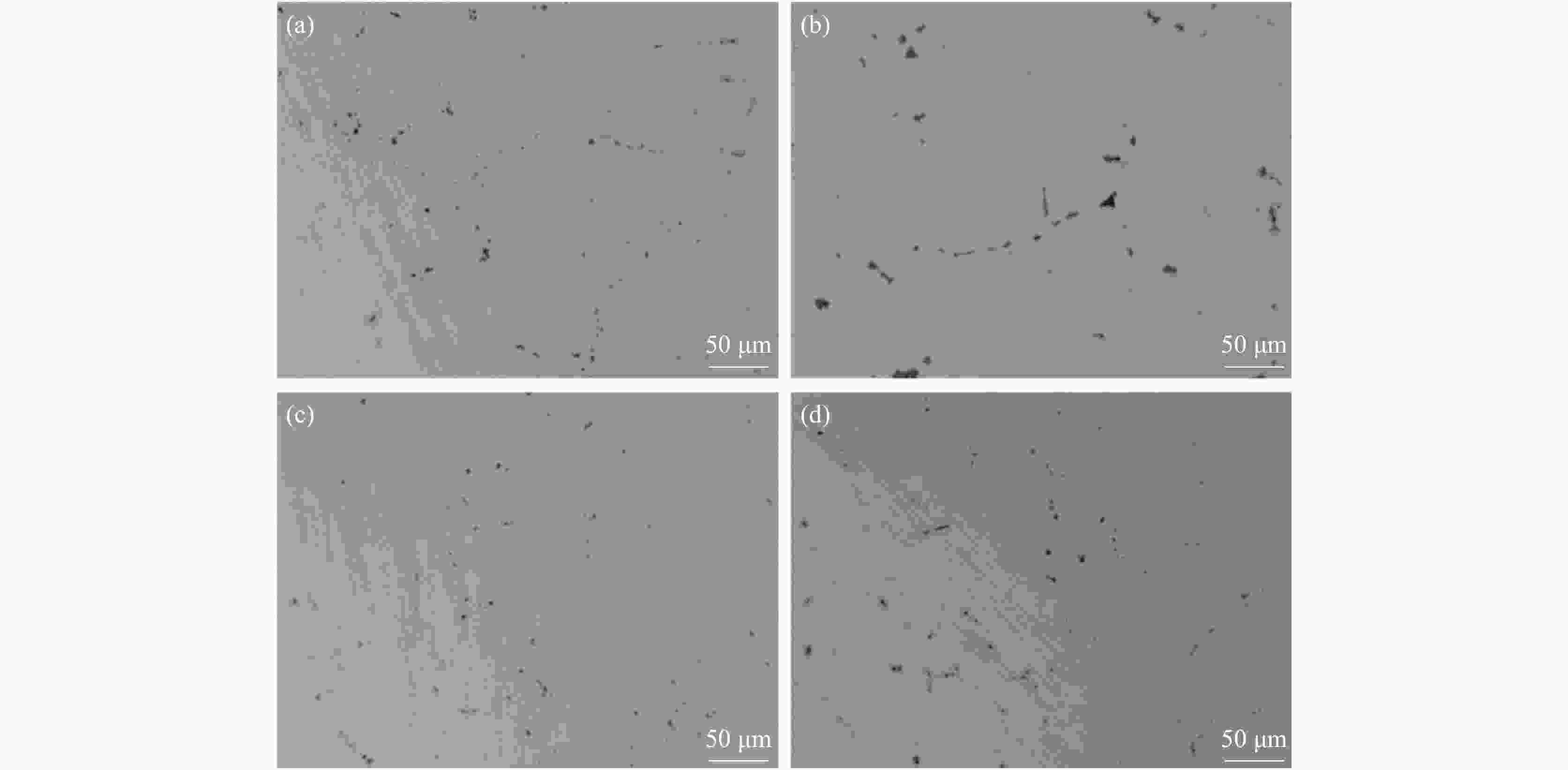
摘要: 基于镁改质非调质钢中夹杂物的形成特征规律,通过计算MgAl2O4与MnS间的错配度以揭示MnS在MgAl2O4上的最佳生长面,以及计算MgAl2O4颗粒之间的主要作用力。结果表明,不添加Mg时,铸坯边部的硫化物主要呈链状沿晶界析出,铸坯中心的硫化物主要呈杆状或角状。当钢中添加Mg后,边部和中心处夹杂物主要呈球状或链状,且尺寸较小。Mg改质后铸坯边部和中心处的夹杂物尺寸均降低,中心区域夹杂物的平均面积由14.33 μm2降低到8.78 μm2,边部区域夹杂物的平均面积由3.17 μm2降低到2.99 μm2,Mg的加入使钢中夹杂物等效面积降低。MnS晶格的(110)晶面和MgAl2O4的(110)晶面晶格错配度为7.65%。腔桥力是MgAl2O4颗粒黏附的作用力,腔桥力约为1×10−8 N。Abstract: Based on the characteristics and formation of the Mg-treated inclusions in non-quenched and tempered steel, the misfit degrees of MgAl2O4+MnS was calculated to reveal the best growth surface and main action forces between the MgAl2O4 inclusions. For Mg free steel, the sulfide in slab edge mainly precipitate in chain shape along grain boundary, while the sulfide in center slab is mainly in rod or angle shape. When Mg is added to the steel, the inclusions at the edge and center of slab are mainly spherical or chain like, and their size are small. The average area of inclusions in center area is reduced from 14.33 μm2 to 8.78 μm2, while in the edge area it is reduced from 3.17 μm2 to 2.99 μm2. Mg addition reduces the equivalent area of inclusions in the steel. The misfit degree between crystal face (110) of MnS and (110) of MgAl2O4 was 7.65%. The main acting force for the attachment of MgAl2O4 was cavity-bridge force of 1×10−8 N.
-
Key words:
- non-quenched and tempered steel /
- Mg /
- MgAl2O4 inclusions /
- misfit degree
-
表 1 F45Mn30非调质钢主要化学成分
Table 1. Main chemical compositions of F45Mn30 non-quenched and tempered steel
% C Si Mn P S Al Mg [O]T 0.456 0.143 1.420 0.0112 0.088 0.007 0.0011 0.0015 表 2 MgAl2O4和MnS晶格错配度计算结果
Table 2. Calculation results for lattice mismatch of MgAl2O4 and MnS inclusions.
[hkl]s [hkl]n d[hkl]s d[hkl]n θ 晶格错配度/% (110)MnS//(110)MgAl2O4 [001] [−1-12] 2.625 2.796 [−111] [−1-10] 4.558 4.915 0 7.65 [−110] [001] 3.695 3.015 (100)MnS//(100)MgAl2O4 [001] [011] 2.256 4.702 [−111] [010] 3.711 8.040 0 16.08 [010] [01-1] 2.190 4.672 (111)MnS//(111)MgAl2O4 [−110] [−110] 3.695 4.682 [−122] [−122] 6.409 9.799 0 31.34 [−101] [−101] 3.695 4.702 -
[1] Xie J B, Zhang D, Yang Q K, et al. Exploration of morphology evolution of the inclusions in Mg-treated 16MnCrS5 steel[J]. Ironmaking and Steelmaking, 2019,6(46):564−573. [2] Guo Dengyang, Wu Xiaodong, Chen Ruilong, et al. Research on precipitation of inclusions in calcium-treated sulfur- containing 20CrMo gear steel[J]. Iron Steel Vanadium Titanium, 2012,33(6):69−73. (郭登仰, 吴晓东, 陈瑞泷, 等. 钙处理含硫20CrMo齿轮钢夹杂物析出研究[J]. 钢铁钒钛, 2012,33(6):69−73. doi: 10.7513/j.issn.1004-7638.2012.06.015 [3] Jiang Z H, Zhang Y, Yang L I, et al. Effect of modification treatment on inclusions in 430 stainless steel by Mg-Al alloys[J]. Journal of Iron and Steel Research International, 2013,20(5):6−10. doi: 10.1016/S1006-706X(13)60089-8 [4] Zhang T S, Wang D Y, Liu C W, et al. Modification of inclusions in liquid iron by Mg treatment[J]. Journal of Iron and Steel Research International, 2014,21(s1):99−103. [5] Wang Junhua, Piao Fengyun, Yu Yongchun, et al. Industrial production process of unquenched steel front axle for heavy automobile[J]. Journal of University of Science and Technology Beijing, 2007,29(S1):105−111. (王俊华, 朴峰云, 于咏春, 等. 重型汽车专用非调质钢前轴工业性生产工艺[J]. 北京科技大学学报, 2007,29(S1):105−111. [6] Luo S J, Su Y H, Lu M J, et al. EBSD analysis of magnesium addition on inclusion formation in SS400 structural steel[J]. Materials Characterization, 2013,82:103−112. doi: 10.1016/j.matchar.2013.05.013 [7] Fan Tian, Yang Qiankun, Xie Jianbo, et al. Effect of Mg on the inclusions in 20CrMo gear steel[J]. Iron Steel Vanadium Titanium, 2019,40(6):149−154. (樊田, 杨乾坤, 谢剑波, 等. 镁对20CrMo齿轮钢中夹杂物的影响[J]. 钢铁钒钛, 2019,40(6):149−154. [8] Ai Kenan, Xie Jianbo, Zeng Zhiqi, et al. Effect of Mg on microstructure and sulfide in non-quenched and tempered steel[J]. Journal of Iron and Steel Research, 2019,31(4):361−367. (艾克南, 谢剑波, 曾志崎, 等. 镁对非调质钢中组织及硫化物的影响[J]. 钢铁研究学报, 2019,31(4):361−367. [9] Xiao Guohua, Dong Han, Wang Maoqiu, et al. Effect of Mg/Ca-treatment on morphology of sulfide in non-quenched and tempered steel[J]. Iron & Steel, 2011,46(4):65−69. (肖国华, 董瀚, 王毛球, 等. 镁和镁钙处理对非调质钢中硫化物形态的影响[J]. 钢铁, 2011,46(4):65−69. [10] Cao L, Wang G C, Yuan X H, et al. Thermodynamics and agglomeration behavior on spinel inclusion in Al-deoxidized steel coupling with Mg treatment[J]. Metals, 2019,9(8):900−911. doi: 10.3390/met9080900 [11] Yuvaloshini J, Shanmugavadivu R, Ravi G. Effect of annealing on optical and structural properties of ZnS/MnS and MnS/ZnS superlattices thin films for solar energy application[J]. Optik International Journal for Light & Electron Optics, 2014,125(6):1775−1779. [12] Krell A, Waetzig K, Klimke J. Influence of the structure of MgO·nAl2O3 spinel lattices on transparent ceramics processing and properties[J]. Journal of the European Ceramic Society, 2012,32(11):3−9. [13] Bramfitt B L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron[J]. Metallurgical and Materials Transactions B, 1970,1(7):1987−1995. [14] Taniguchi S, Kikuchi A. Mechanisms of collision and coagulation between fine particles in fluid[J]. Tetsu-to-hagane, 1993,78(4):527−535. [15] Sasai K. Interaction between alumina inclusions in molten steel due to cavity bridge force[J]. ISIJ International, 2016,56(6):1013−1022. doi: 10.2355/isijinternational.ISIJINT-2016-038 [16] Chan D, Henry J, White L R. The interaction of colloidal particles collected at fluid interfaces[J]. Journal of Colloid and Interface Science, 1981,79(2):410−418. doi: 10.1016/0021-9797(81)90092-8 [17] Bratko D, Curtis R A, Blanch H W, et al. Interaction between hydrophobic surfaces with metastable intervening liquid[J]. Journal of Chemical Physics, 2001,115(8):3873−3877. doi: 10.1063/1.1386926 [18] Lei Shaolong, Jiang Min, Yang Die, et al. Effect of oxides on MnS precipitation in aluminum-deoxidized steel[J]. Journal of University of Science and Technology Beijing, 2013,35(11):1443−1449. (雷少龙, 姜敏, 杨叠, 等. Al脱氧钢中氧化物对MnS析出的影响[J]. 北京科技大学学报, 2013,35(11):1443−1449. -




 下载:
下载: