Study on the effect of molten salt electro-deoxidation on the preparation of titanium metal
-
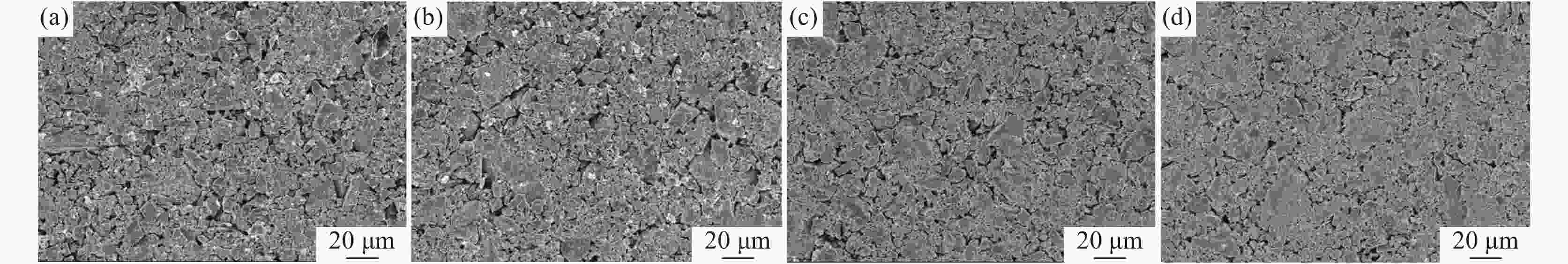
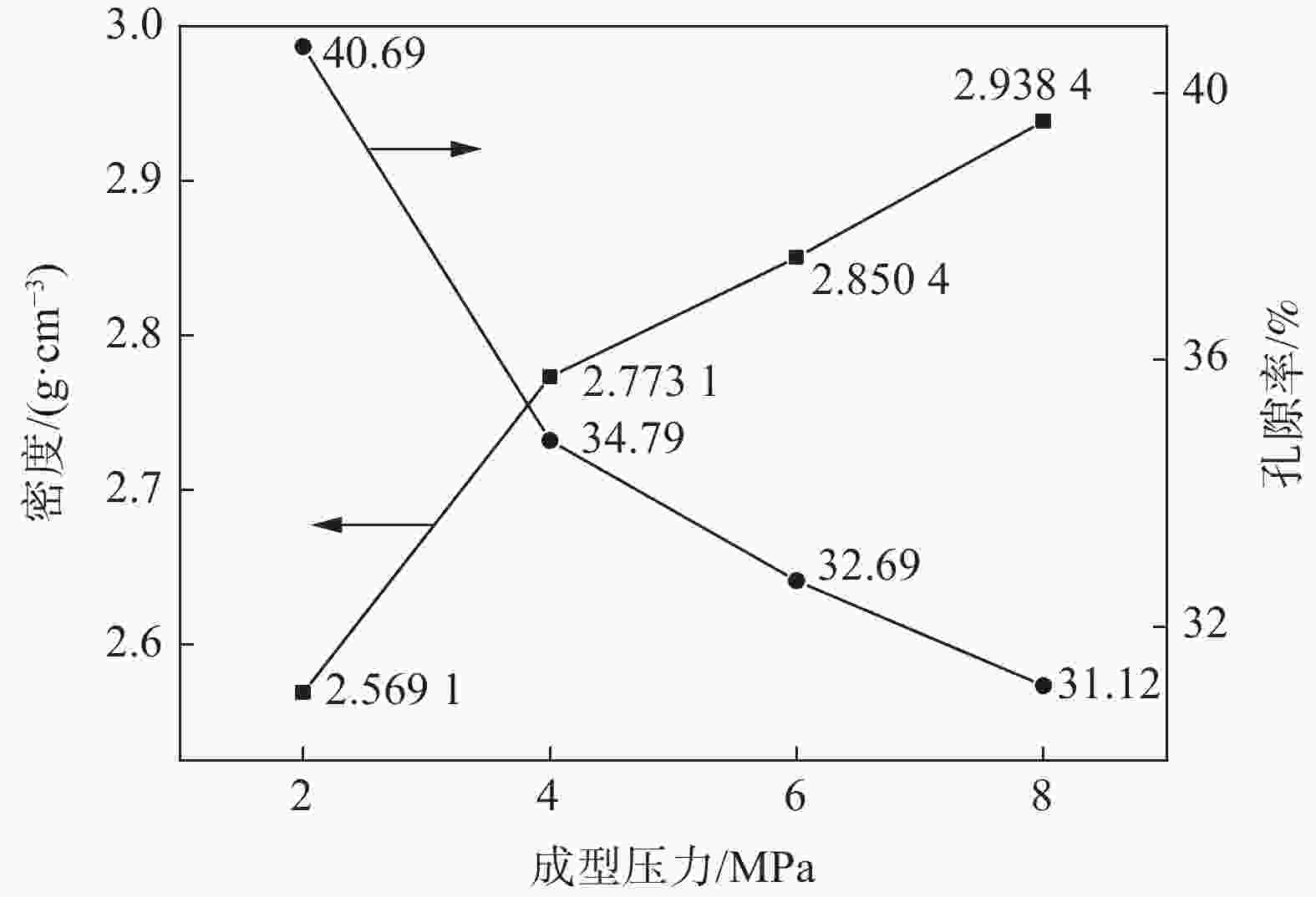
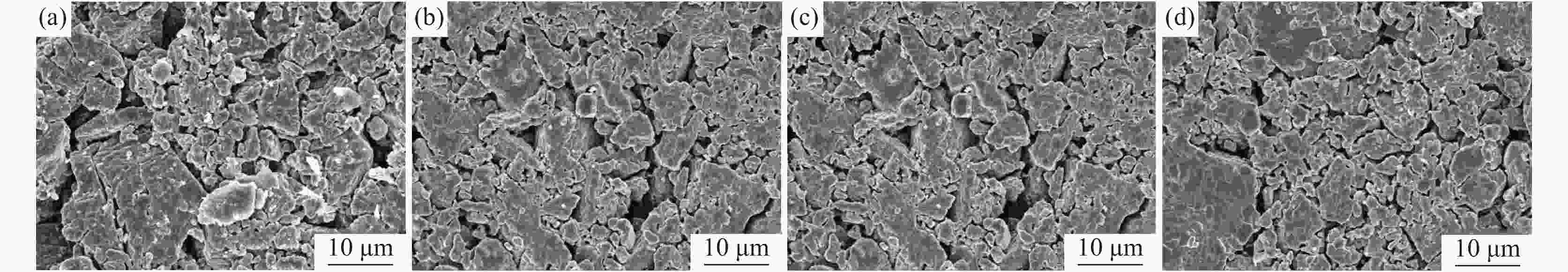
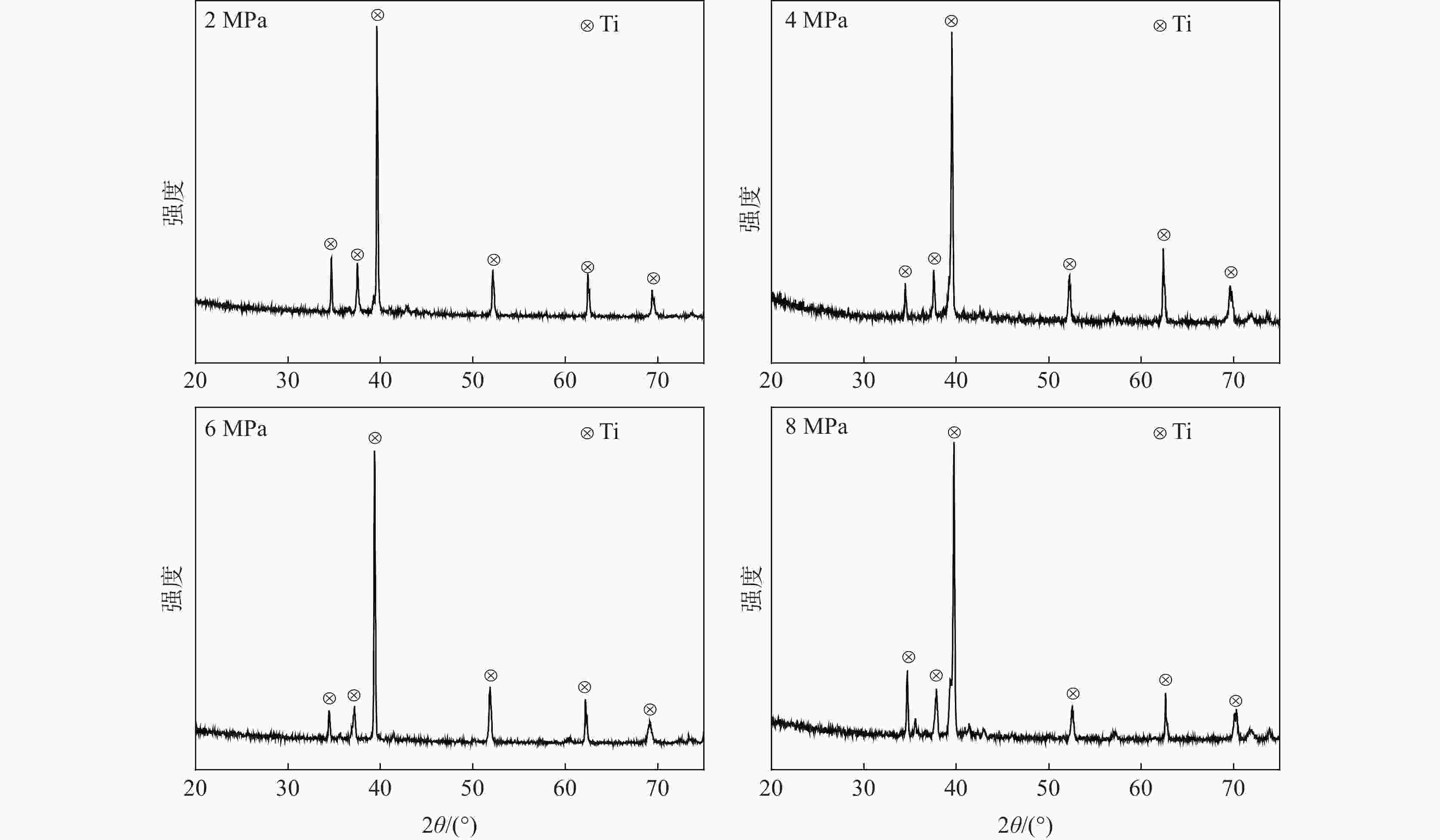
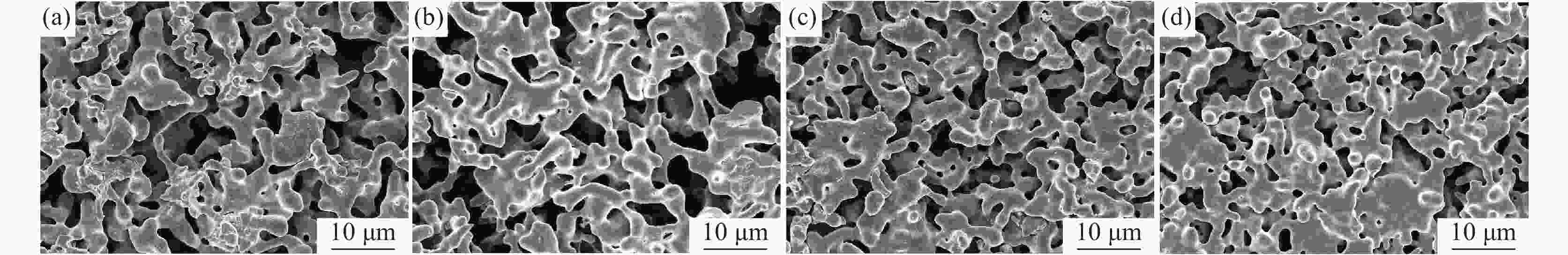
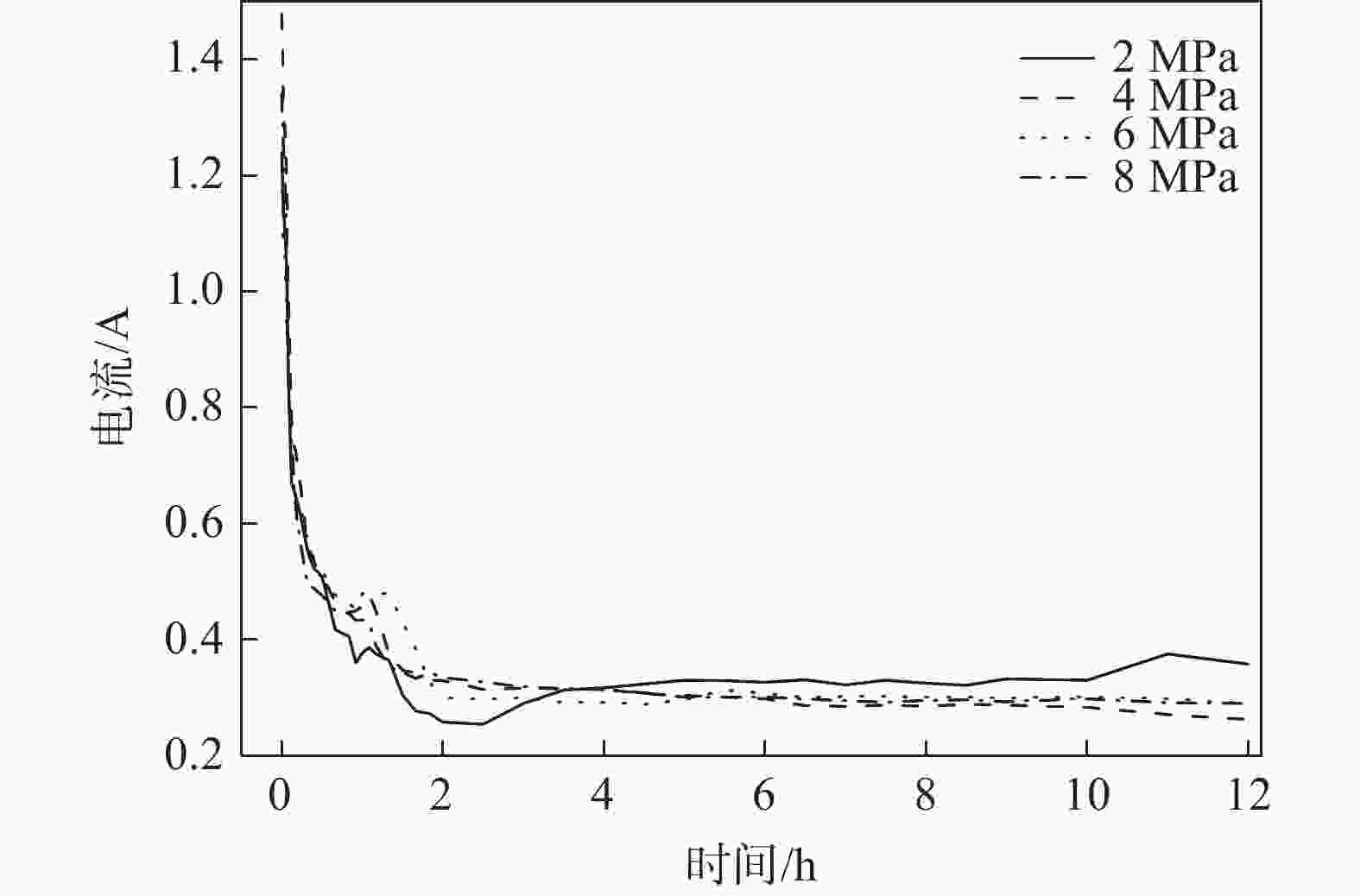
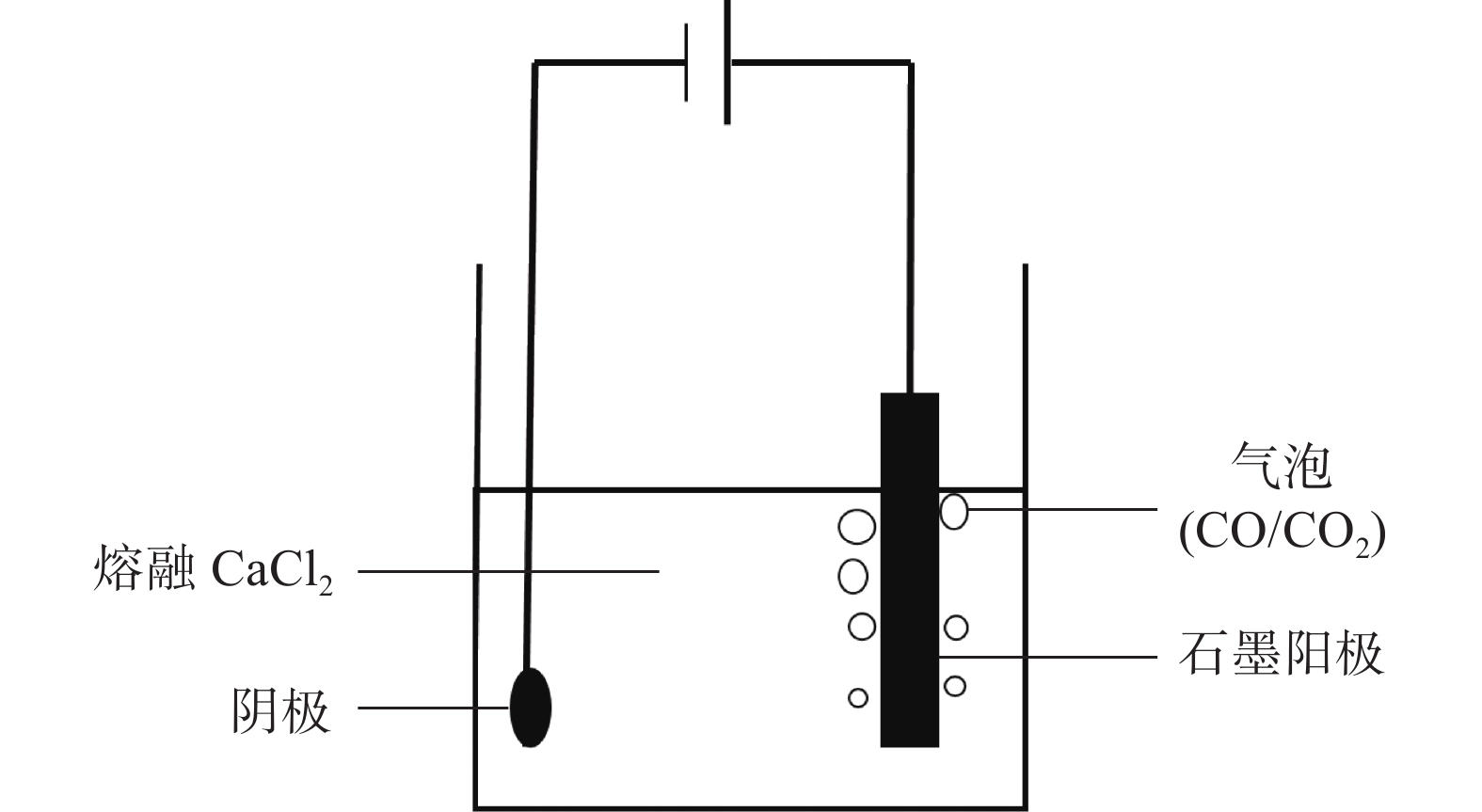
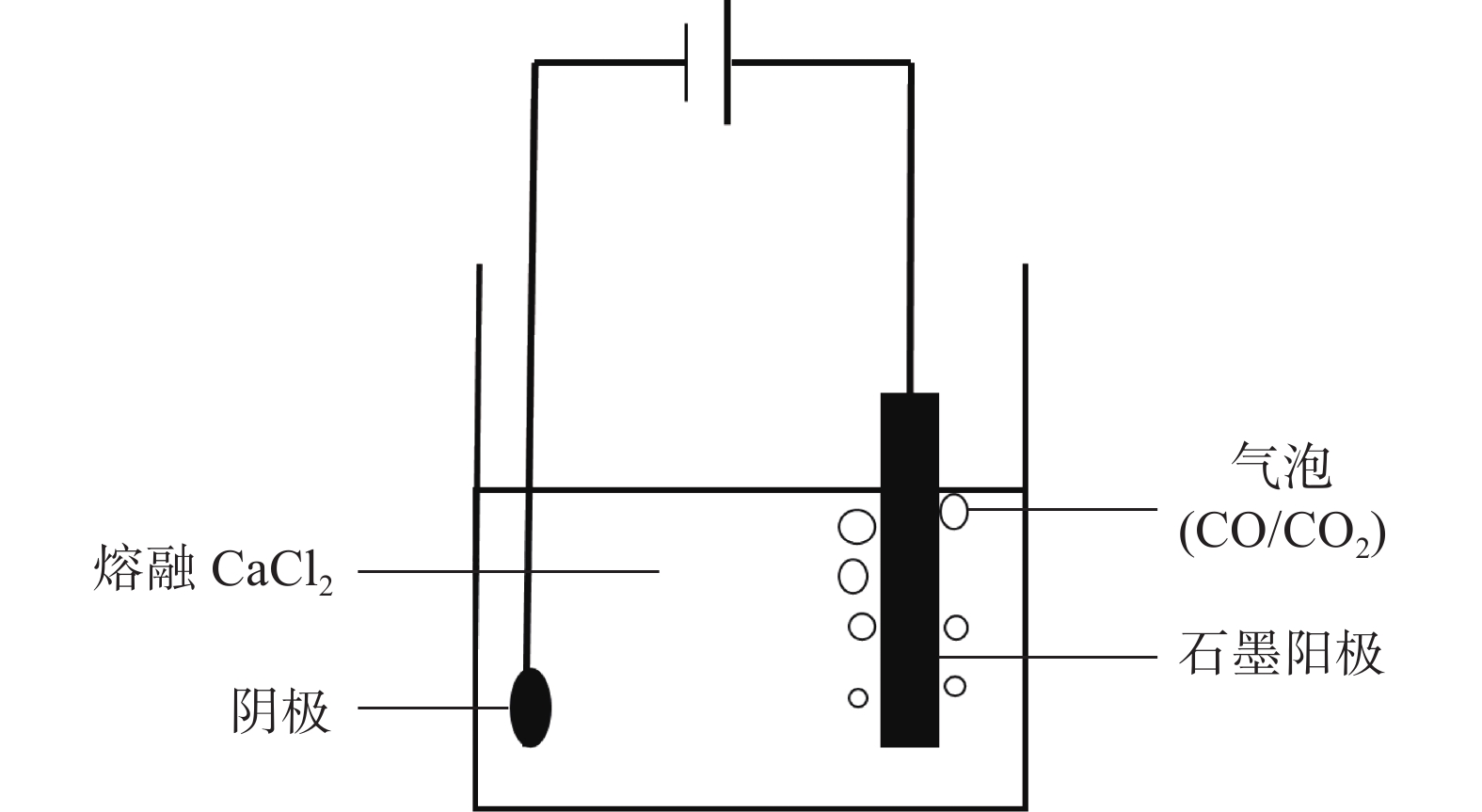
摘要: 在高纯氩气气氛下,在CaCl2熔盐中电解高钛渣制备金属钛,研究了成型压力与阴极片孔隙率的关系以及对电解过程的影响,并采用 XRD、 SEM等分析手段对阴极片及电解后的物相和微观形貌结构进行表征。结果表明:成型压力对阴极片孔隙率有直接影响,随着成型压力升高,阴极孔隙率下降;阴极片的孔隙率直接影响电脱氧过程,适当的孔隙率有利于形成中间产物CaTiO3和提高电还原速率。4 MPa压制的阴极1050 ℃烧结2 h,孔隙率为34.79%,电解12 h 产物氧含量降低至1.75%,钛含量为95.72%,此时阴极片的电化学性能较好。Abstract: Titanium metal was prepared by electrolysis of high titanium slag in CaCl2 molten salt under a high-purity argon atmosphere. The relationship between forming pressure and porosity of cathode sheet as well as the influence on the electrolysis process were studied. The phases and microstructures of the cathode sheet and that after electrolysis were characterized by XRD and SEM. The results showed that the forming pressure had a direct influence on the porosity of the cathode sheet, which decreased with the increase of the forming pressure. The porosity of the cathode sheet directly affected the electrode deoxidation process, and an appropriate porosity was beneficial to the formation of intermediate product CaTiO3 and the increase of the electroreduction rate. The porosity of the cathode pressed by 4 MPa is 34.79% when sintered at 1 050 ℃ for 2 h, and the oxygen content of the product is reduced to 1.75% and titanium content is 95.72% when electrolyzed for 12 h, which shows a better electrochemical performance of the cathode sheet.
-
表 1 高钛渣化学成分
Table 1. Chemical compositions of the high titanium slag
% O Al Si Ti Fe Ca 47.45 2.26 1.94 38.53 8.61 1.21 表 2 不同压力电解后的阴极化学成分
Table 2. Chemical composition of the cathodes after electrolysis under different pressure
成型压力/MPa w/% O Al Si Ti Fe Ca 2 5.43 0.71 1.09 90.56 1.71 0.50 4 1.75 0.49 0.76 95.72 0.80 0.48 6 3.96 0.45 1.03 93.14 0.92 0.54 8 4.29 0.57 1.05 93.06 0.47 0.56 表 3 各处理过程的杂质元素含量
Table 3. Impurity element content of various treatment processes
mg Fe Al Si 杂质总量 172.2 45.2 38.8 CaCl2熔盐 110.41 36.21 26.54 HCl洗涤液 35.12 5.32 6.24 尾气吸收液 1.2 0.62 0.53 -
[1] Zhang Xiaowei, Zhang Wanyi, Tong Ying, et al. Current status and utilization trends of global titanium ore resources[J]. Mineral Conservation and Utilization, 2019,39(5):68−75. (张晓伟, 张万益, 童英, 等. 全球钛矿资源现状与利用趋势[J]. 矿产保护与利用, 2019,39(5):68−75. [2] Guo Li, He Weixia, Zhou Peng, et al. Research status and development prospect of titanium and titanium alloy products in China[J]. Thermal Processing Technology, 2020,49(22):22−28. (郭鲤, 何伟霞, 周鹏, 等. 我国钛及钛合金产品的研究现状及发展前景[J]. 热加工工艺, 2020,49(22):22−28. [3] Jia Hong, Lu Fusheng, Hao Bin. Report on the development of titanium industry in China in 2020[J]. Iron Steel Vanadium Titanium, 2021,42(3):1−9. (贾翃, 逯福生, 郝斌. 2020年中国钛工业发展报告[J]. 钢铁钒钛, 2021,42(3):1−9. [4] Chen G Z, Fray D J, Farthing T W. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride[J]. Nature, 2000,407(2):361−364. [5] Liu S. A study of preparation of titanium metal by the electrochemical reduction of titanium dioxide in molten salt[J]. Procedia Earth & Planetary Science, 2011,2:1−6. [6] Liu Z W, Zhang H L, Pei L L, et al. Direct electrolytic preparation of chromium metal in CaCl2 –NaCl eutectic salt[J]. Transactions of Nonferrous Metals Society of China, 2018,28(2):376−384. doi: 10.1016/S1003-6326(18)64671-0 [7] Barnett R P, Fray D J. Electro-deoxidation of Ta2O5 in calcium chloride–calcium oxide melts[J]. Journal of Materials Science, 2014,49(12):4148−4160. doi: 10.1007/s10853-014-8110-x [8] Wang Lin. Study on the preparation of magnesium-zirconium alloy by molten salt electrolysis[J]. Journal of Yunnan University for Nationalities( Natural Science Edition), 2014,23(6):420−423. (王琳. 熔盐电解制备镁锆合金工艺研究[J]. 云南民族大学学报(自然科学版), 2014,23(6):420−423. [9] 李泽全. TiO2熔盐电解制备钛及其反应机理的研究[D]. 重庆: 重庆大学, 2011.Li Zequan. Electrolytic preparation of titanium from TiO2 molten salt and its reaction mechanism[D]. Chongqing: Chongqing University, 2011. [10] Xu Yidong, Lu Ping, Tang Yajun. Study on the preparation of silicon powder by molten salt electrolysis[J]. Non-ferrous Metals:Smelting Part, 2012,(9):69−71. (徐义东, 卢平, 唐亚军. 熔盐电解法制备硅粉的研究[J]. 有色金属:冶炼部分, 2012,(9):69−71. [11] Li Zhen, Sun Jianke, Chang Pengbei, et al. Study on the performance of TiO2 electrode in the preparation of titanium metal by molten salt electrolysis[J]. Materials Direct, 2011,25(2):60−62. (李珍, 孙建科, 常鹏北, 等. 熔盐电解法制备金属钛工艺中TiO2电极性能研究[J]. 材料导报, 2011,25(2):60−62. [12] Zhang Zhen, Hua Yixin, Xu Cunying, et al. Preparation of TiC/SiC nanocomposite powders by electrolysis of high titanium slag/C with CaO-CaCl2-NaCl molten salt[J]. Rare Metals, 2018,(4):408−414. (张臻, 华一新, 徐存英, 等. CaO-CaCl2-NaCl熔盐电解高钛渣/C制备TiC/SiC纳米复合粉体[J]. 稀有金属, 2018,(4):408−414. [13] 申园园. 熔盐电解含钛废渣制备金属钛及杂质行为[D]. 贵阳: 贵州大学, 2018.Shen Yuanyuan. Preparation of titanium metal from titanium-containing waste slag by molten salt electrolysis and impurity behavior [D]. Guiyang: Guizhou University, 2018. [14] 邢伟. 熔盐电脱氧法由高钛渣制备高钛铁合金[D]. 沈阳: 东北大学, 2009.Xing Wei. Preparation of high titanium iron alloy from high titanium slag by molten salt electro-deoxidation [D]. Shenyang: Northeastern University, 2009. [15] 况文浩. 熔盐电解高钛渣制备钛硅合金的研究[D]. 昆明: 昆明理工大学, 2017.Kuang Wenhao. Study on the preparation of titanium-silicon alloy by molten salt electrolysis of high titanium slag[D]. Kunming: Kunming University of Science and Technology, 2017. [16] Li Qiang, Zhao Hui, Huo Lihua, et al. Electrode properties of Sr doped La2CuO4 as new cathode material for intermediate-temperature SOFCs[J]. Electrochemistry Communications, 2007,9(7):1508−1512. doi: 10.1016/j.elecom.2007.02.013 [17] Xu B, Hong Y S, Mohassab Y, et al. Correction: Structures, preparation and applications of titanium suboxides[J]. RSC Advances, 2016,6(114):112737. doi: 10.1039/C6RA90124G [18] Liu Xuyang, Hu Meilong, Bai Chenguang, et al. Effect of electrical conductivity and porosity of cathode on electro-deoxidation process of ilmenite concentrate[J]. Rare Metal Materials & Engineering, 2017,46(5):1176−1182. [19] Chen Hualin, Wang Zhiyong, Jin Xianbo, et al. An ion diffusion model for solid oxide cathode processes and its validation by electrolysis of Ta2O5 molten salt[J]. Electrochemistry, 2014,(3):266−271. (陈华林, 王志勇, 金先波, 等. 固态氧化物阴极过程的离子扩散模型及其Ta2 O5熔盐电解验证[J]. 电化学, 2014,(3):266−271. [20] Schwandt C, Fray D J. Determination of the kinetic pathway in the electrochemical reduction of titanium dioxide in molten calcium chloride[J]. Electrochimica Acta, 2006,51(1):66−76. -




 下载:
下载: