Preparation and anti-corrosion properties of photoresponsive titanium based nano-films
-
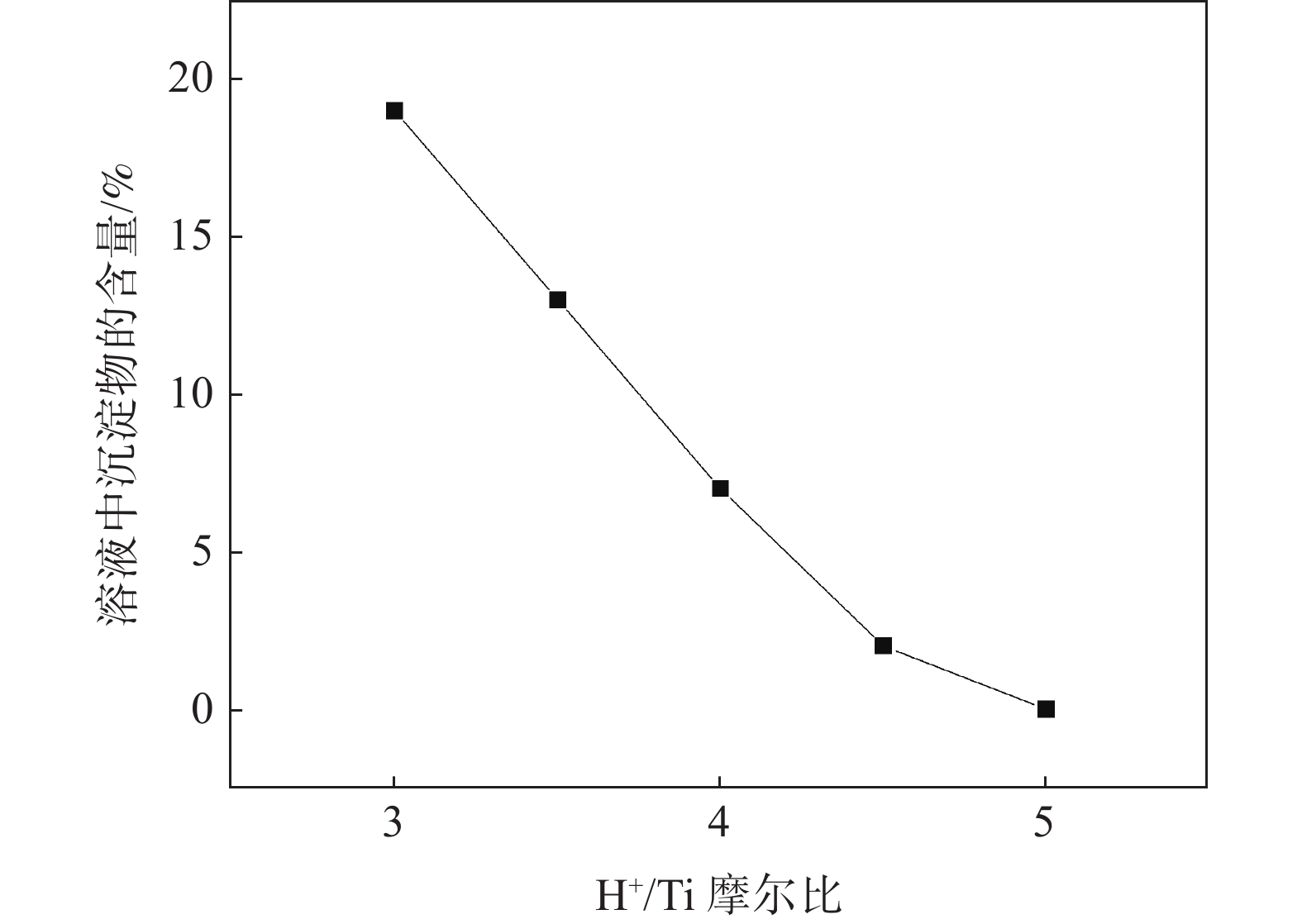
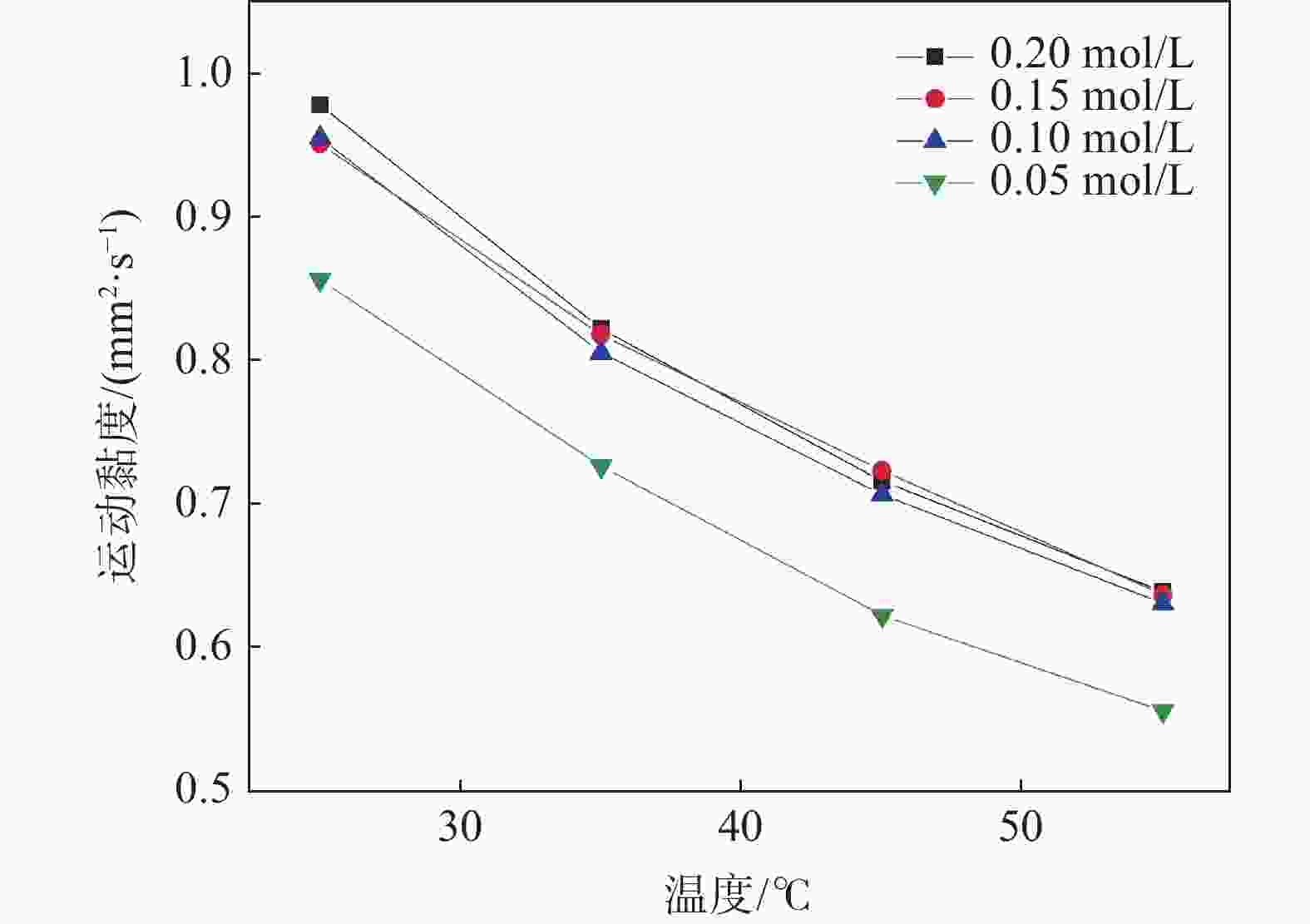
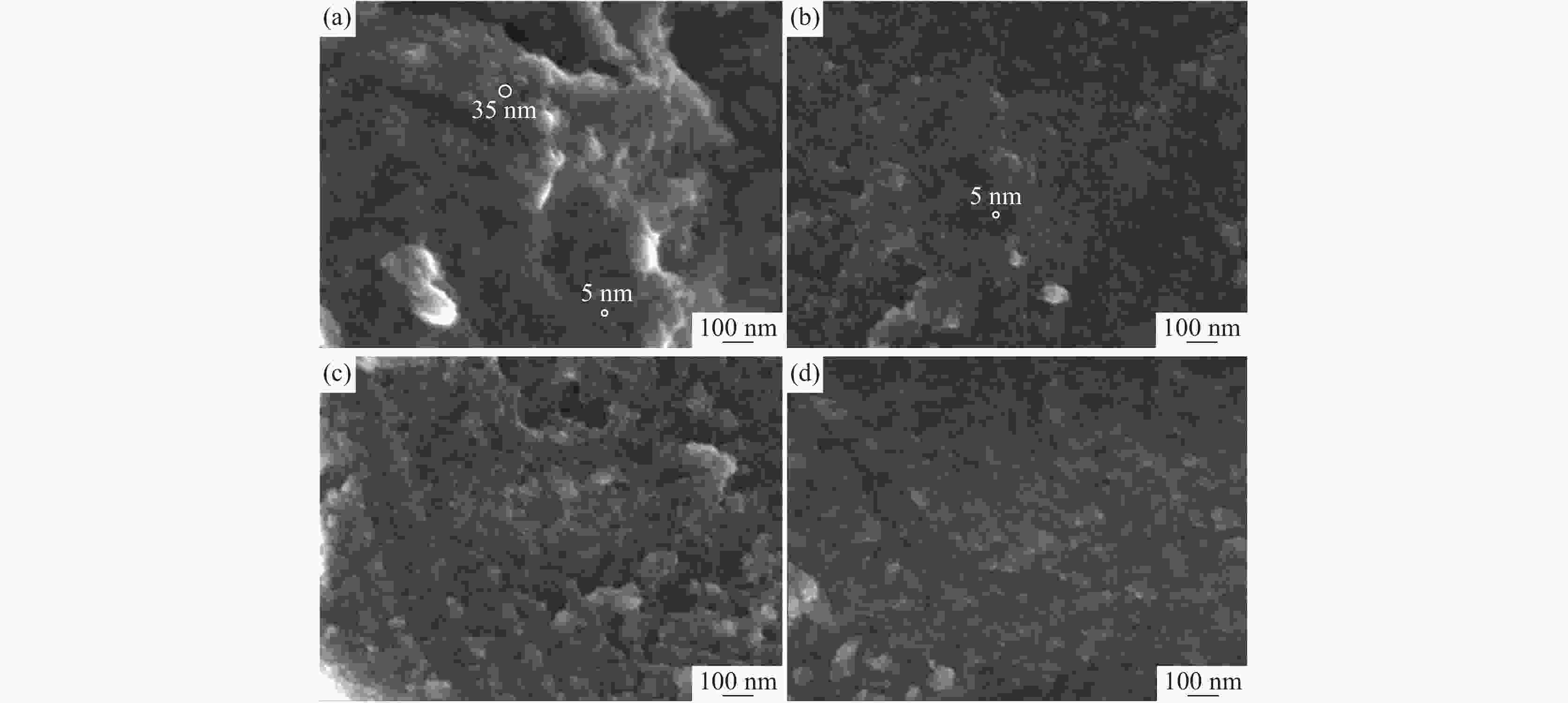
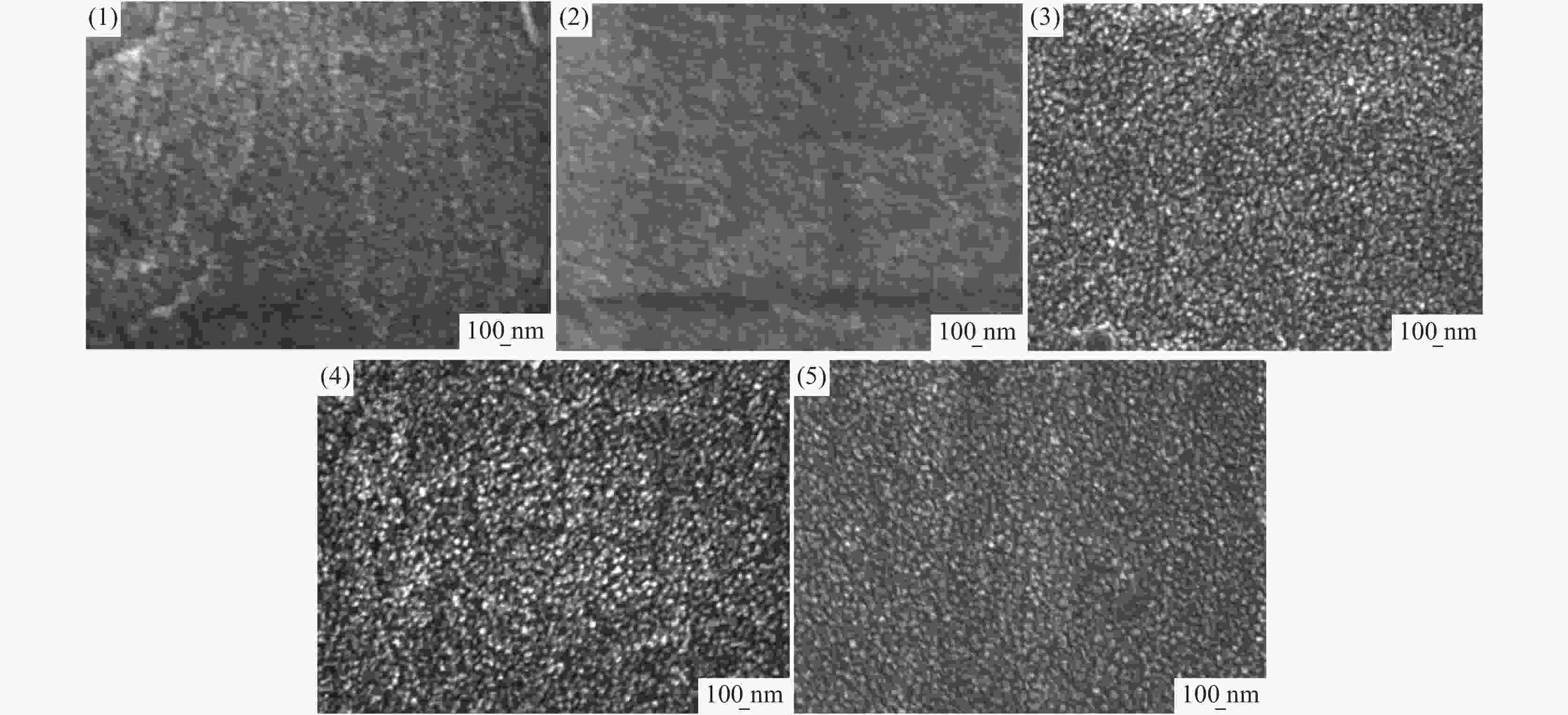
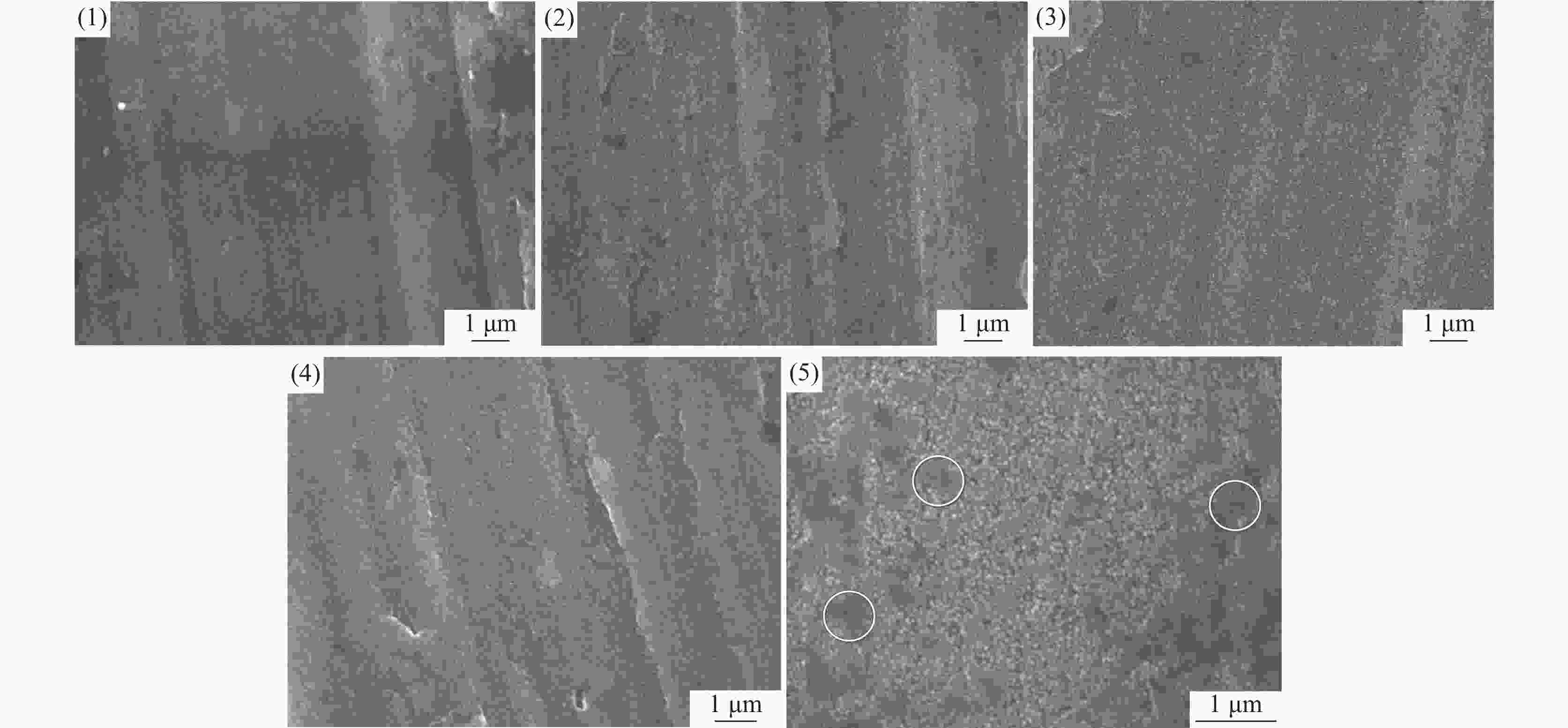
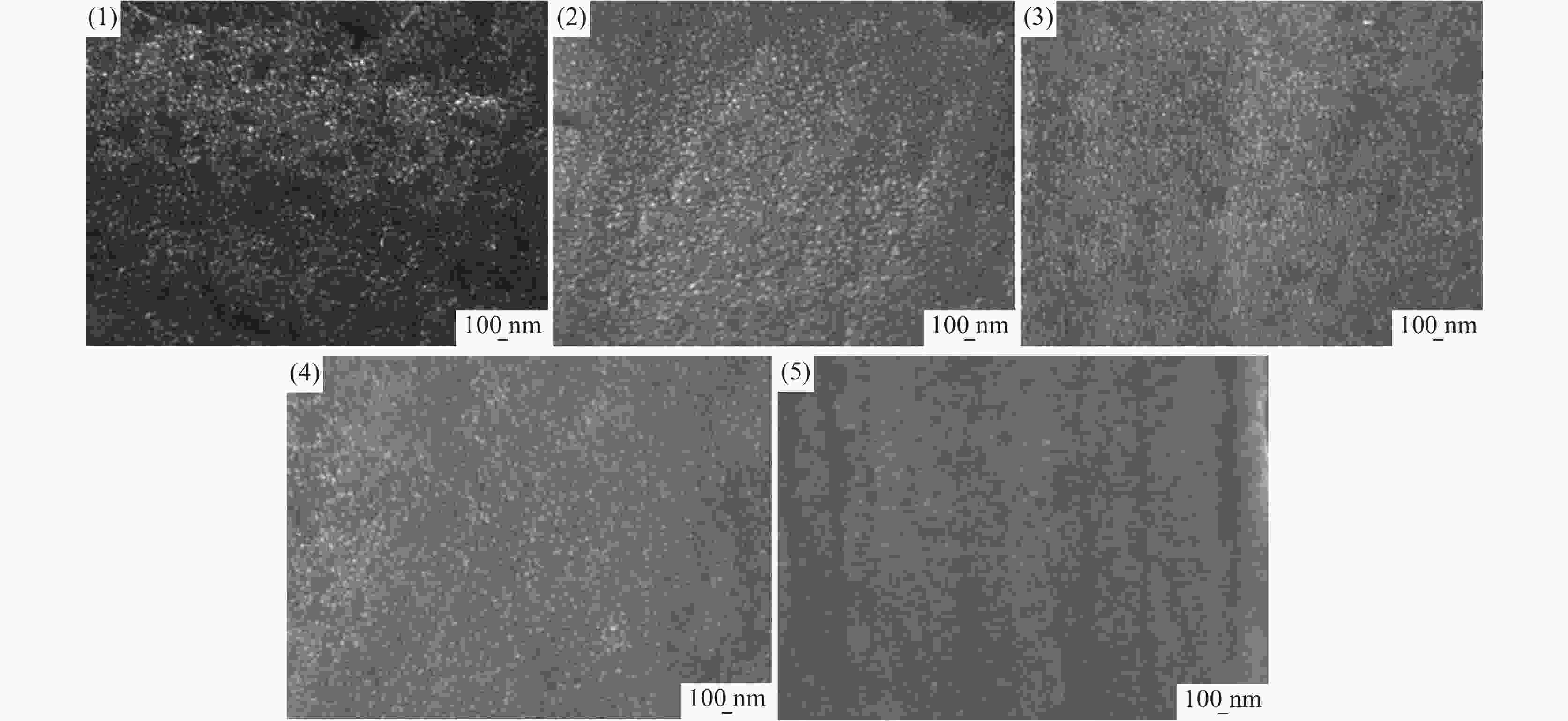
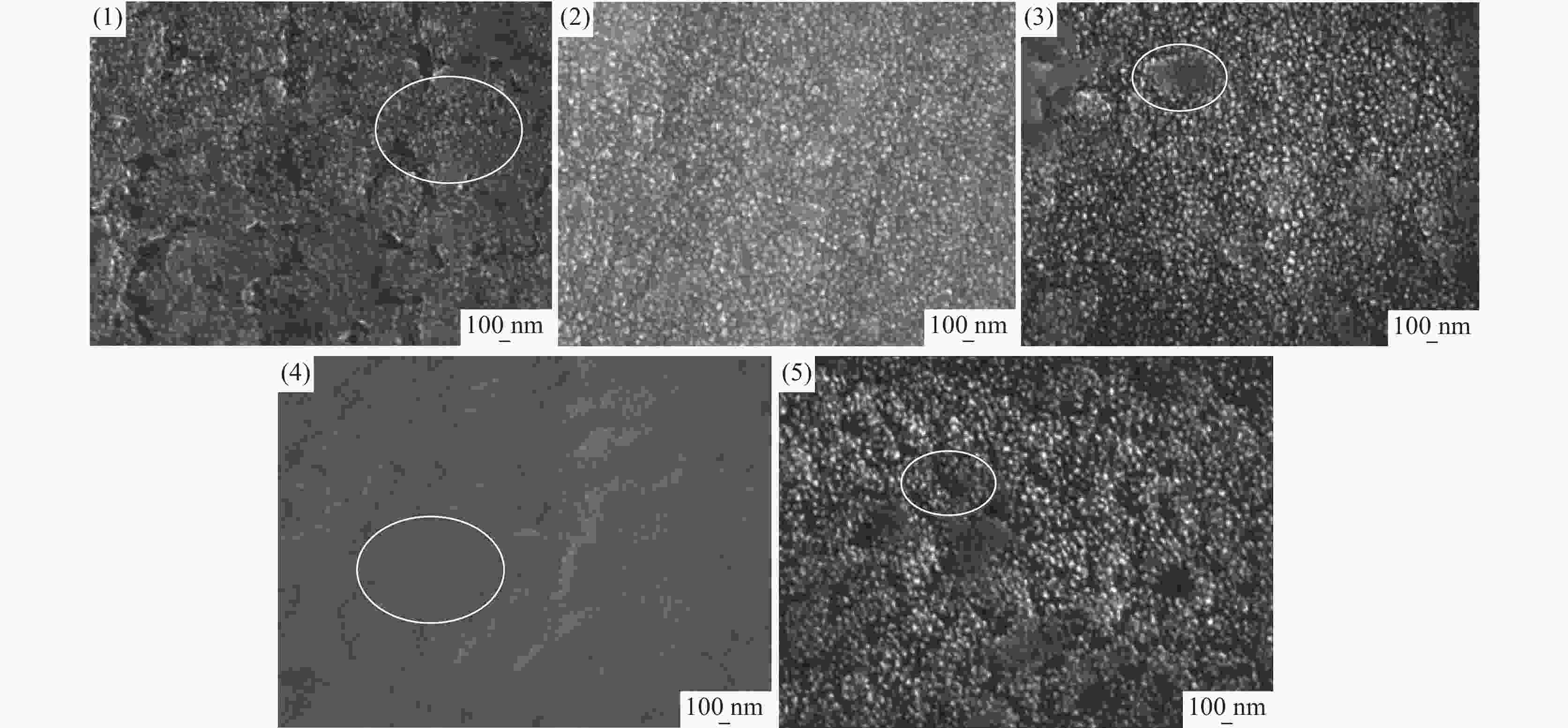
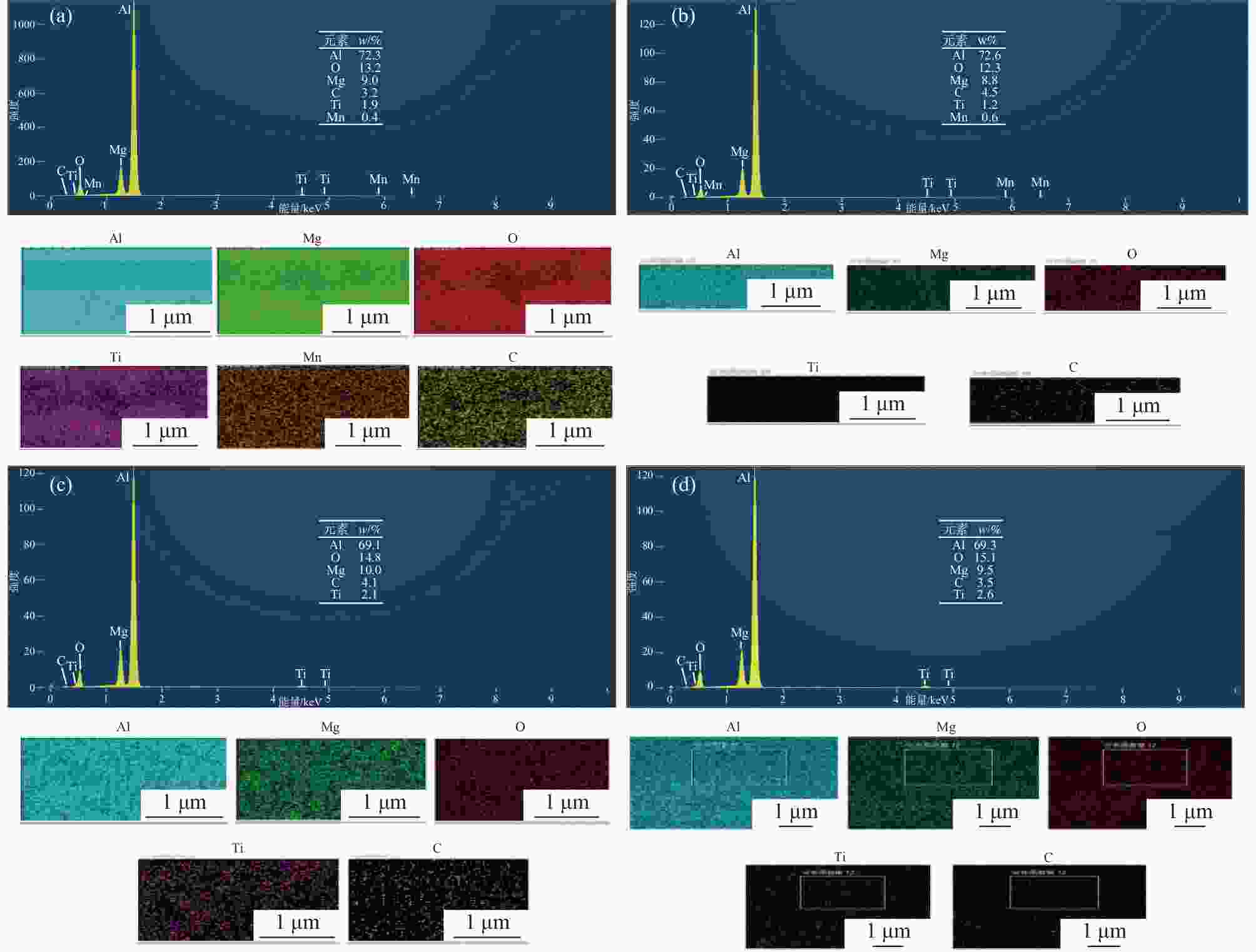
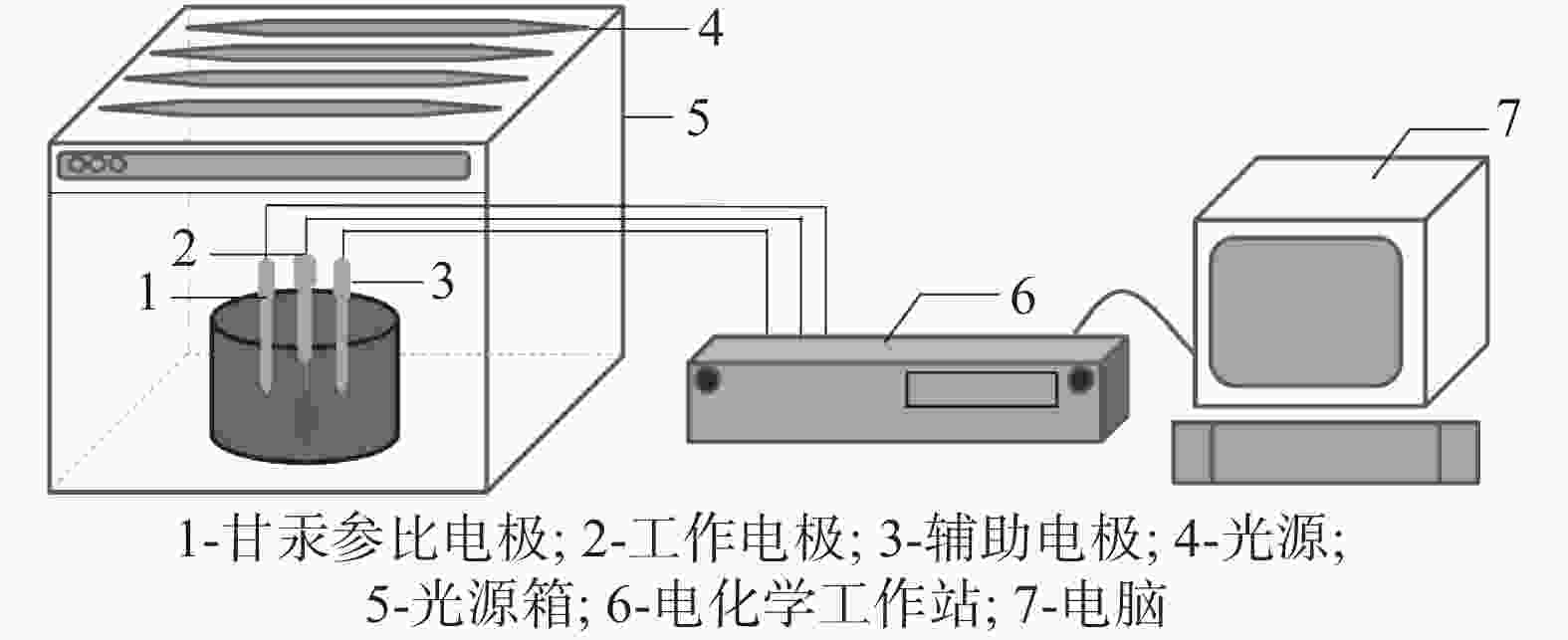
摘要: 基于企业生产研究背景与优势,提出利用廉价且易得的钛源(例如:TiOSO4)材料为原料,采用反应简单、操作便捷的sol-gel法在基材表面制备一层或多层纳米级钛基薄膜,研究镀膜后基材在紫外光光照和暗态条件下的耐腐蚀能力,意图通过镀膜大幅度提高基材的耐腐蚀能力。通过选择钛源、沉淀剂、水溶胶的钛浓度、络合剂比例和反应温度优化水溶胶制备工艺;同时研究不同浓度水溶胶的粘温特性及采用水溶胶浓缩为干凝胶的方法对水溶胶中颗粒粒径和形貌进行观测,溶胶粒径均小于50 nm。后期采用匀速提拉法在基板表面进行不同层数薄膜的制备,采用相应热处理得到纳米钛基薄膜,并研究纳米薄膜的形貌。最后将基片制作成电极浸渍在3.5%氯化钠溶液中测试其防腐性能,制备纳米薄膜表面颗粒粒径均小于50 nm,五层膜厚度在1 μm左右。防腐性能测试结果表明在基材表面制备防腐薄膜提高了基材腐蚀电位、降低其腐蚀电流,在暗态条件下,相较基板的防护效率最高可达99.73%;在紫外光照下,相较基板的防护效率最高可达99.14%。同时通过暗态和紫外光照下的基板的开路电位分析,在紫外光照射下相对于暗态下的开路电位出现不同程度负移的情况,展现出薄膜具有光响应性。Abstract: Based on the research background and advantages of enterprise production, cheap and easily available titanium sources (such as TiOSO4) were used as raw materials to prepare one or more nano-titanium-based films on the substrate surface by sol-gel method with simple reaction and convenient operation. The corrosion resistance of substrate after coating was studied under ultraviolet light and dark state conditions, with the intention of greatly improving the corrosion resistance of the substrate through the coating. The preparation process of hydrosol was optimized by selecting titanium source, precipitant, titanium concentration of hydrosol, proportion of complexing agent and reaction temperature. At the same time, the viscosity and temperature characteristics of hydrosol at different concentrations were studied, and the size and morphology of the particles were observed by the sol-gel method. The particle size of the sol was less than 50 nm. In the later stage, the films with different layers were prepared on the substrate surface by uniform pulling method, the nano titanium based films were obtained by corresponding heat treatment, and the morphology of the nano films was studied. Finally, the substrate was made into an electrode and immersed in a 3.5% sodium chloride solution to test its anti-corrosion performance. The particle size on the surface of the nano-film was less than 50 nm, and the thickness of the five layers of film was 1 μm. The results show that the corrosion resistance of the substrate can be improved by anti-corrosive film on the substrate surface. The protection efficiency is up to 99.73% and 99.14% respectively in dark state and under ultraviolet light, compared with that of the substrate. Meanwhile, through the analysis of the open circuit potential of the substrate under dark state and ultraviolet light, the negative shift of the open circuit potential of the substrate under ultraviolet light is different from that of the dark state, which shows the light response of the film.
-
Key words:
- nano-titanium-based film /
- sol-gel /
- titanium-based hydrosol /
- anti-corrosive /
- TiOSO4 /
- light response
-
表 1 试验试剂
Table 1. Experimental reagents
试剂名称 分子式 生产厂家 纯度 硫酸氧钛 TiOSO4·H2O 天津市光复精细化工研究所 分析纯 氨水 NH4OH 成都市科隆化学品有限公司 分析纯 硝酸 HNO3 重庆川东化工集团有限公司 分析纯 无水乙醇 C2H5OH 成都金山化学试剂有限公司 分析纯 表 2 以硫酸氧钛为原料的薄膜覆盖的基片对应暗态和紫外光下开路电位
Table 2. Open circuit potentials of thin film-covered substrates prepared with TiOSO4 in dark state and under UV light
样品编号 暗态OCP/V 紫外光照态OCP/V A-1 −0.173 −0.224 A-2 −0.165 −0.187 A-3 −0.18 −0.191 A-4 −0.129 −0.163 A-5 −0.149 −0.187 B-1 −0.168 −0.175 B-2 −0.155 −0.155 B-3 −0.14 −0.166 B-4 −0.17 −0.173 B-5 −0.195 −0.197 C-1 −0.211 −0.218 C-2 −0.197 −0.212 C-3 −0.202 −0.219 C-4 −0.194 −0.209 C-5 −0.176 −0.205 D-1 −0.26 −0.273 D-2 −0.219 −0.227 D-3 −0.163 −0.197 D-4 −0.178 −0.205 D-5 −0.216 −0.235 -
[1] Tao Qi, Li Fenfang, Xing Jianmin. Research progress of metal corrosion and its protective measures[J]. Hunan Nonferrous Metals, 2007,(2):48−51. (陶琦, 李芬芳, 邢健敏. 金属腐蚀及其防护措施的研究进展[J]. 湖南有色金属, 2007,(2):48−51. [2] 王成. 不锈钢表面电化学处理及耐腐蚀机理研究[D]. 厦门: 厦门大学, 2018.Wang Cheng. Study on electrochemical treatment and corrosion resistance mechanism of stainless steel surface[D]. Xiamen: Xiamen University, 2018. [3] 彭叔森, 赵文杰, 曾志翔, 等. 304不锈钢表面有机硅耐蚀薄膜的制备及其防腐性能[C]//第九届全国表面工程大会暨第四届全国青年表面工程论坛论文集. 宁波: 中国机械工程学会, 2012.Peng Shusen, Zhao Wenjie, Zeng Zhixiang, et al. Preparation and corrosion resistance of 304 Stainless steel surface silicone film[C]//Proceedings of the 9 th National Surface Engineering Conference and the 4 th National Youth Surface Engineering Forum. Ningbo: Chinese Society of Mechanical Engineering, 2012. [4] Liu Xiyan, Jiang Jianming, Chen Zhengtao, et al. Research progress in anti-corrosive protection for aluminum alloy[J]. Modern Paint & Coating, 2007,10(12):11−14. (刘希燕, 蒋健明, 陈正涛, 等. 铝合金防腐保护研究进展[J]. 现代涂料与涂装, 2007,10(12):11−14. doi: 10.3969/j.issn.1007-9548.2007.12.004 [5] Zhu Y, Zhang L, Gao C, et al. The synthesis of nanosized TiO2 powder using a sol-gel method with TiCl4 as a precursor[J]. Journal of Materials Science, 2000,35(16):4049−4054. doi: 10.1023/A:1004882120249 [6] 吴震弘. TiO2薄膜在不锈钢上防腐机理的研究及应用[D]. 杭州: 浙江理工大学, 2014.Wu Zhenhong. Study and application of TiO2 film anticorrosion mechanism on stainless steel[D]. Hangzhou: Zhejiang Sci-tech University, 2014. [7] Koelsch M, Cassaignon, Guillemoles J F. Comparison of optical and electrochemical properties of anatase and brookite TiO2 synthesized by the sol–gel method[J]. Thin Solid Films, 2002,403:312−319. [8] Yu J, Zhao X, Zhao Q. Effect of surface structure on photocatalytic activity of TiO2 thin films prepared by sol-gel method[J]. Thin Solid Films, 2000,379(1-2):7−14. doi: 10.1016/S0040-6090(00)01542-X [9] Tian Shouwei, Wang Zuohui, Liu Yangsi, et al. Preparation of photocatalytic self-cleaning ceramics supported by nano-TiO2 thin films[J]. Iron Steel Vanadium Titanium, 2006,27(3):26−30. (田守卫, 王作辉, 刘阳思, 等. 负载纳米TiO2薄膜的光催化自清洁陶瓷的制备[J]. 钢铁钒钛, 2006,27(3):26−30. doi: 10.3969/j.issn.1004-7638.2006.03.006 [10] Zhang Wenbin, Zhou Yan. Progress in photocatalysis of nano-TiO2[J]. Iron Steel Vanadium Titanium, 2005,26(4):26−33. (张文彬, 周燕. 纳米TiO2光催化研究进展[J]. 钢铁钒钛, 2005,26(4):26−33. doi: 10.3969/j.issn.1004-7638.2005.04.006 [11] Wu Jianchun. Application of rutile nano-titanium dioxide in coatings[J]. Iron Steel Vanadium Titanium, 2021,42(1):7. (吴健春. 金红石纳米二氧化钛在涂料中的应用[J]. 钢铁钒钛, 2021,42(1):7. [12] Wu Jianchun, Lu Ruifang. Effect of rutile nano-TiO2 on UV resistance of paint[J]. Iron Steel Vanadium Titanium, 2015,36(3):4. (吴健春, 路瑞芳. 金红石纳米TiO2对油漆抗紫外性能的影响[J]. 钢铁钒钛, 2015,36(3):4. [13] Bautista-Ruiz J, Aperador W, Delgado A, et al. Synthesis and characterization of anticorrosive coatings of SiO2 -TiO2 - ZrO2 obtained from sol-gel suspensions[J]. International Journal of Electrochemical Science, 2014,9(8):4144−4157. [14] Kim S J, Ko J Y. Electrochemical properties of Al and Al alloys relevant to corrosion protection in seawater environments[J]. Korean Journal of Chemical Engineering, 2006,23(5):847−853. doi: 10.1007/BF02705939 [15] Zheng Fuyang, Ding Hongbo, Ma Tingchun, et al. Study of lithium anticorrosive film on aluminum alloy[J]. Electroplating & Finishing, 2000,(4):4−6. [16] Abreu N M. Low and high temperature aqueous alteration of the matrices of CR chondrites: Nano-SEM, EPMA, and TEM study [C]//43 nd Annual Lunar and Planetary Science Conference, 2012. [17] Salge T, Terborg R, Ball A D, et al. Advanced SEM/EDS analysis using an annular silicon drift detector (SDD): Applications in nano, life, earth and planetary sciences below micrometer scale[C]//Proceedings of the 18 th International Microscopy Conference, 2014. [18] Ma Yufang. Analysis of electrochemical corrosion and electrochemical analysis problems[J]. Science and Technology Toget Rich Guide, 2014,(29):155. (马玉芳. 浅析电化学腐蚀和电化学分析问题[J]. 科技致富向导, 2014,(29):155. [19] Cao Jingyi, Zhang Feng. Electrochemical rapid evaluation method for corrosion resistance of coatings[J]. Modern Paint and Finishing, 2009,12(12):51−52. (曹京宜, 张锋. 涂层防腐蚀性能电化学快速评价方法[J]. 现代涂料与涂装, 2009,12(12):51−52. doi: 10.3969/j.issn.1007-9548.2009.12.016 [20] Chen Y, Chen X, Liu T, et al. Effect of potential on electrochemical corrosion behavior of 316 L stainless steel in borate buffer solution[J]. Journal of Chinese Society for Corrosion & Protection, 2015,35(2):137−143. [21] Zhou Qionghua. Electrochemical study on corrosion resistance of coated metals[J]. Journal of Electric Power Science and Technology, 2001,16(1):16−80. (周琼花. 涂装金属防蚀性能的电化学研究[J]. 电力科学与技术学报, 2001,16(1):16−80. -





 下载:
下载: