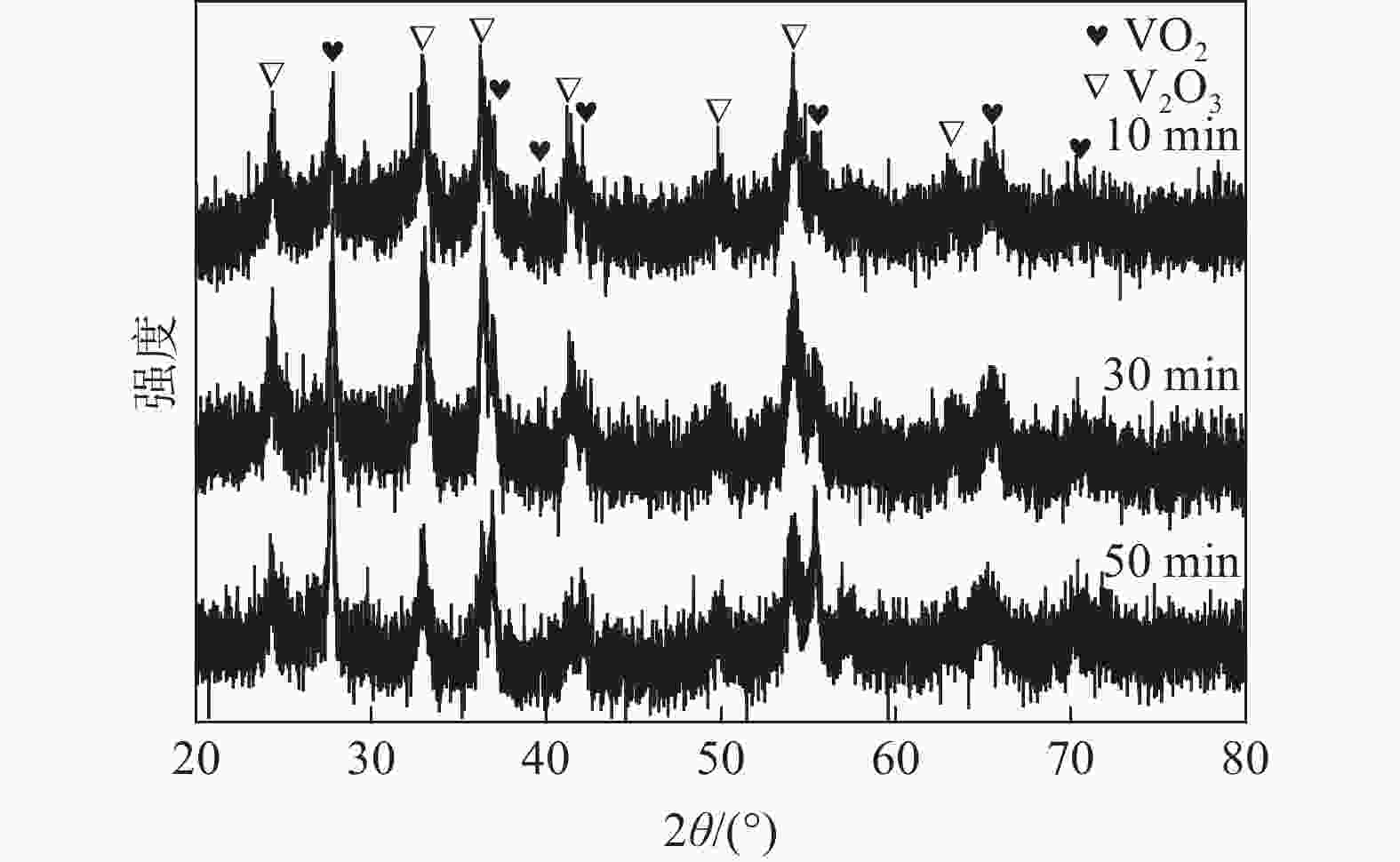
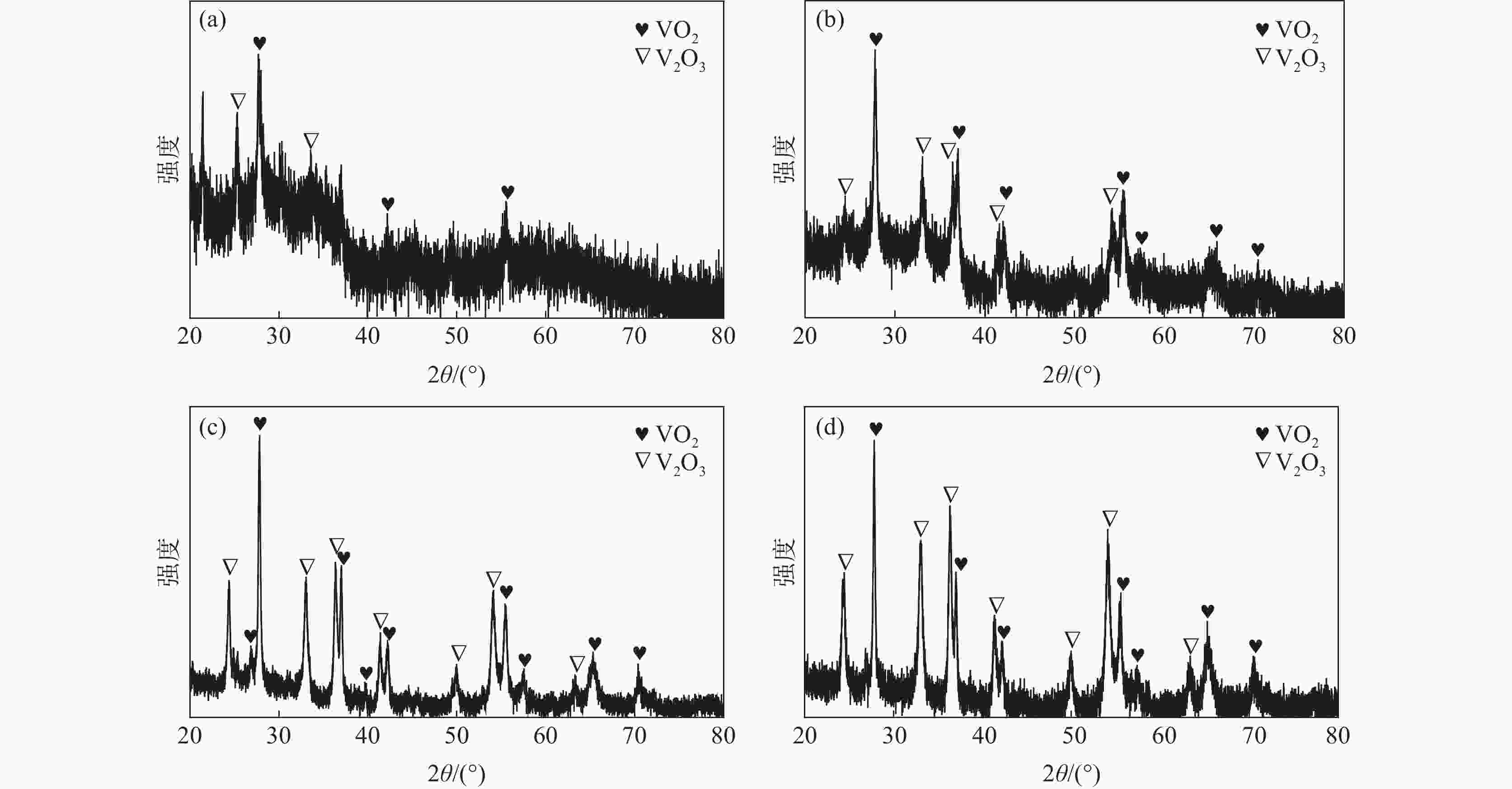
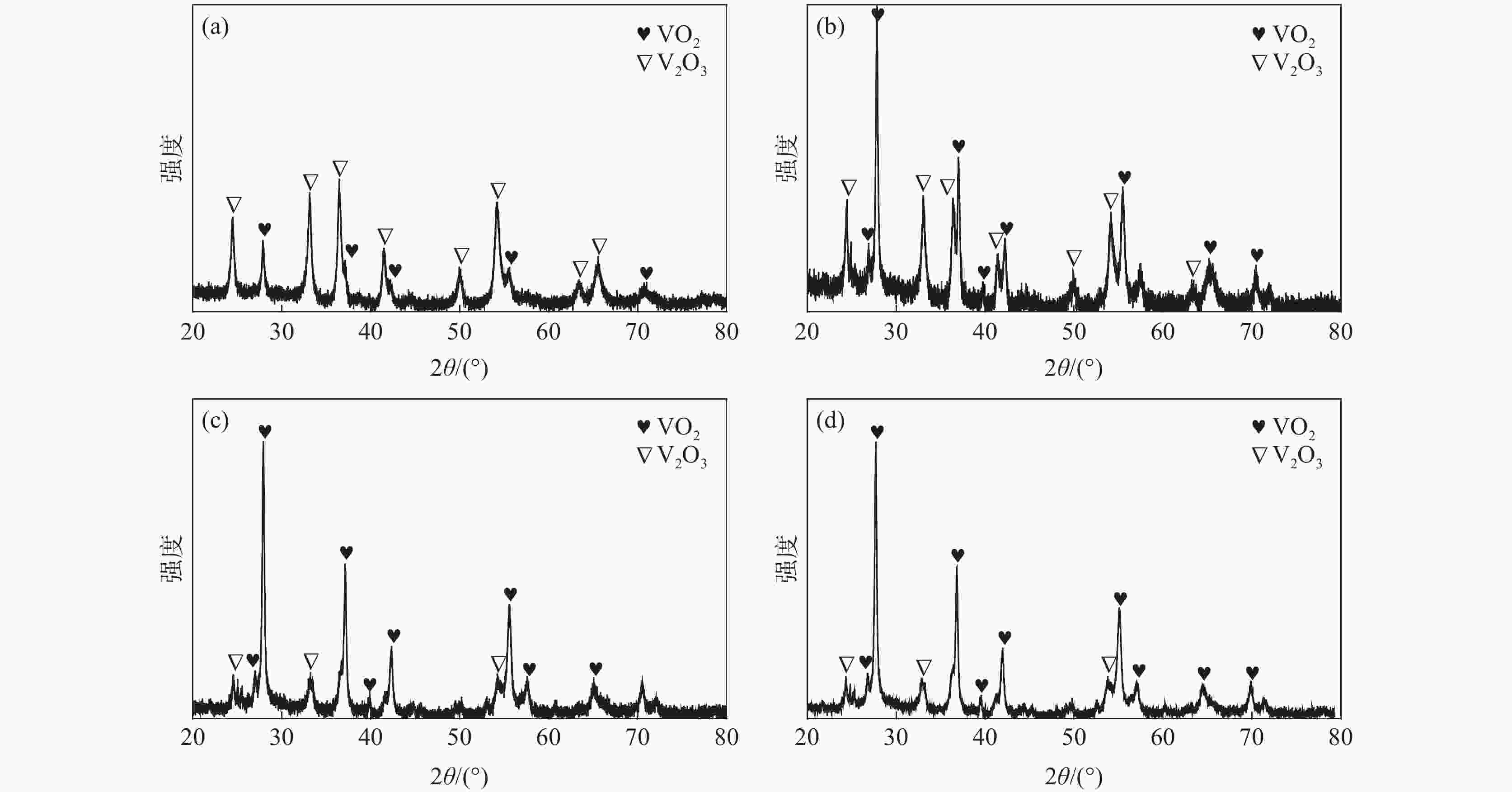
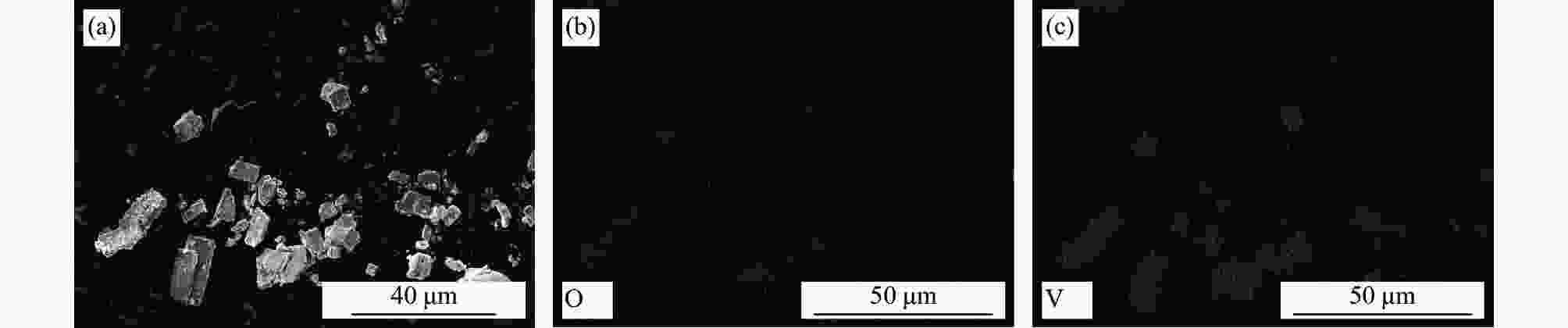
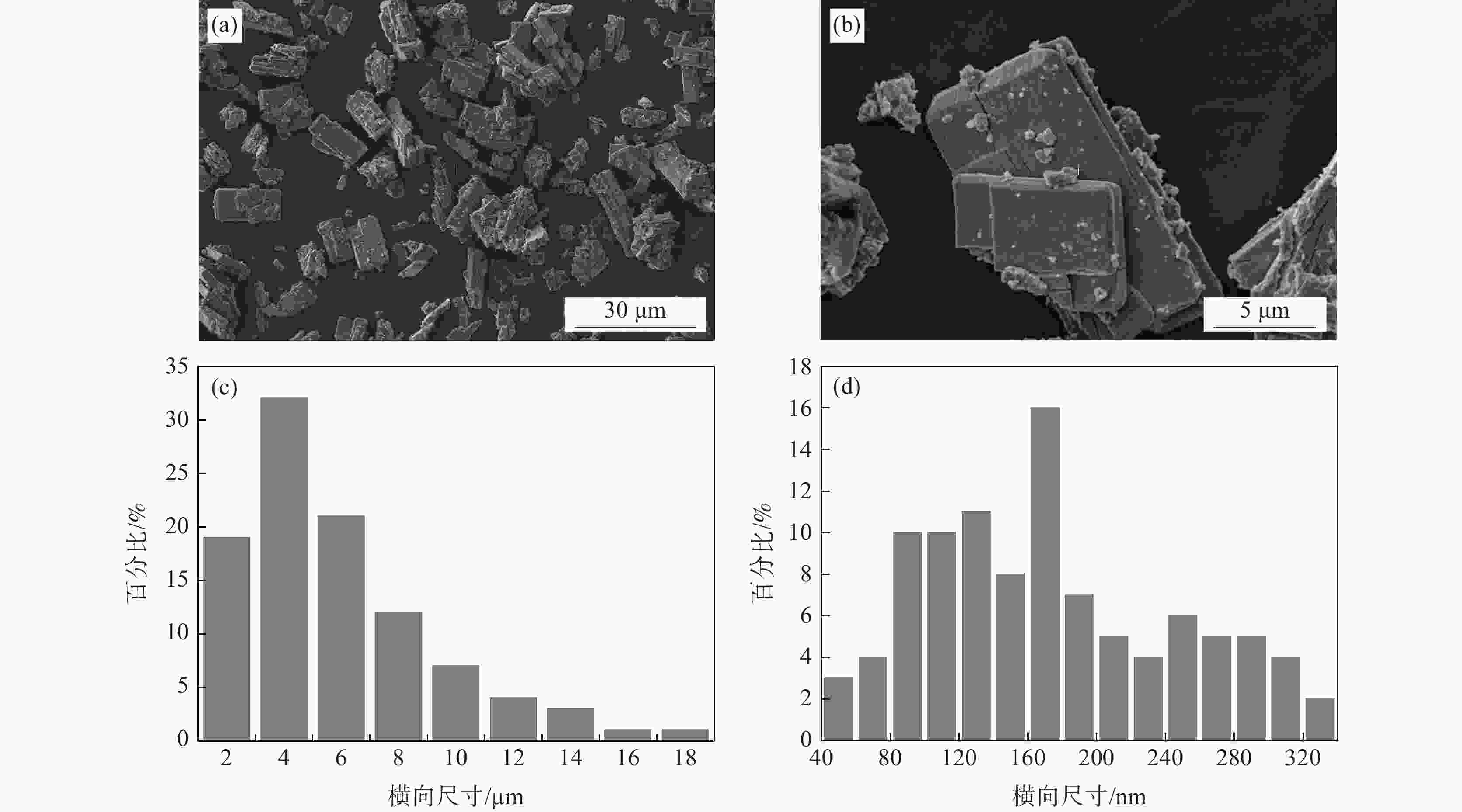
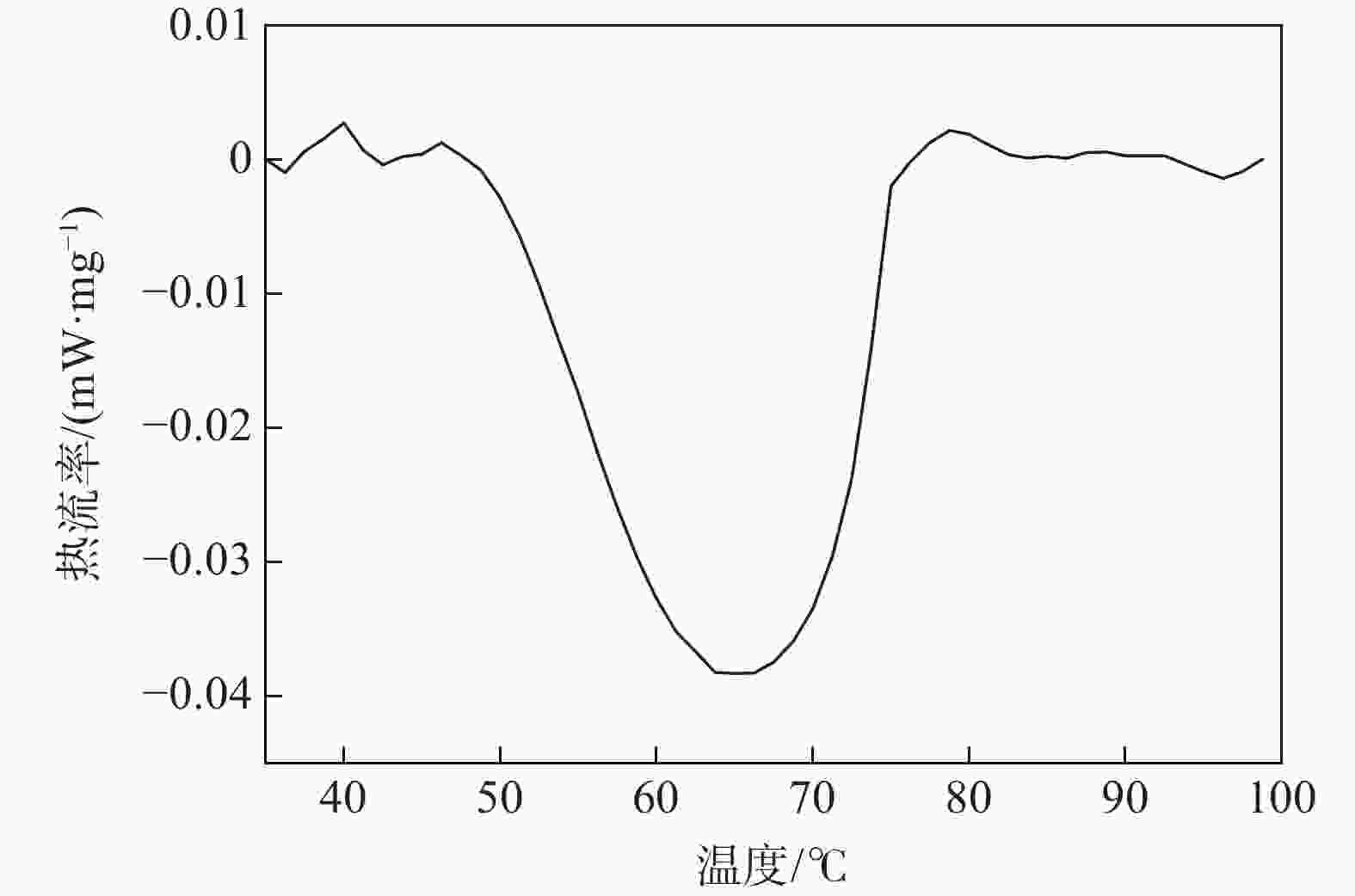
| [1] |
Wang Shufen, Liu Minsu, Kong Lingbing, et al. Recent progress in VO2 smart coatings: Strategies to improve the thermochromic properties[J]. Progress in Materials Science, 2016,81:1−54. doi: 10.1016/j.pmatsci.2016.03.001
|
| [2] |
Li Kaibin, Li Ming, Xu Chang, et al. VO2(M) nanoparticles with controllable phase transition and high nanothermochromic performance[J]. Journal of Alloys and Compounds, 2019,11:5602.
|
| [3] |
Zhu Guang, Huo Yuehua, Shi Yanqiong. Switchable broadband terahertz absorber based on temperature control[J]. Laser & Optoelectronics Progress, 2021,58(13):1316001. (朱广, 霍跃华, 史艳琼. 基于温度控制的可切换宽带太赫兹吸波器[J]. 激光与光电子学进展, 2021,58(13):1316001.
|
| [4] |
Negm Ayman, Bakr Mohamed, Howlader Matiar, et al. Switching plasmonic resonance in multi-gap infrared metasurface absorber using vanadium dioxide patches[J]. Smart Materials and Structures, 2021,30(7):075011. doi: 10.1088/1361-665X/abfb86
|
| [5] |
Liu Ying. Xu Xiang. Hydrogen and sodium ions co-intercalated vanadium dioxide electrode materials with enhanced zinc ion storage capacity[J]. Nano Energy, 2021,86:106124. doi: 10.1016/j.nanoen.2021.106124
|
| [6] |
Morin F J. Oxides which show a metal-to-insulator transition at the neel temperature[J]. Physical Review Letters, 1959,3:34−36. doi: 10.1103/PhysRevLett.3.34
|
| [7] |
Leroux C, Nihoul G, Tendeloo G V. From VO2(B) to VO2(R): Theoretical structures of VO2 polymorphs and in situ electron microscopy[J]. Phys. rev. b, 1998,57:5111−5121.
|
| [8] |
Wen Zeng, Chen Nan, Xie Weiguang. Research progress on the preparation methods for VO2 nanoparticles and their application in smart windows[J]. Cryst Eng. Comm., 2020,22:851−869. doi: 10.1039/C9CE01655D
|
| [9] |
Amador-Alvarado S, Flores-Camacho J M, Solís-Zamudio A, et al. Temperature-dependent infrared ellipsometry of Mo-doped VO2 thin films across the insulator to metal transition[J]. Scientific Reports, 2020, 10: 8555.
|
| [10] |
Luo Juan, Hu Fangrong, Li Guangyuan. Broadband switchable terahertz half-quarter-wave plate based on VO2-metal hybrid metasurface with over underdamped transition[J]. Journal of Physics D: Applied Physics , 2021, 54: 505111.
|
| [11] |
Yi Jing, Yan Wenbin, Zhang Xiaojun, et al. Hydrothermal synthesis of nano vanadium oxide powder[J]. Fine Chemicals, 2016,33(4):361−365. (易静, 颜文斌, 张晓君, 等. 水热法制备纳米二氧化钒粉体[J]. 精细化工, 2016,33(4):361−365.
|
| [12] |
Liu Bo, Peng Sui, Chen Yong, et al. Effect of chemical precipitation process on particle size of VO precursor and its hydrothermal crystallization[J]. Iron Steel Vanadium Titanium, 2020,41(5):58−65. (刘波, 彭穗, 陈勇, 等. 化学沉淀过程对VO2前驱体粒径的影响及其水热晶化的研究[J]. 钢铁钒钛, 2020,41(5):58−65.
|
| [13] |
Jongbae Kim, Lee Donguk Lee, Yeo Inyeok, et al. Hydrothermal synthesis of monoclinic vanadium dioxide nanocrystals using phase-pure vanadium precursors for high-performance smart windows[J]. Solar Energy Materials and Solar Cells, 2021,226:111055. doi: 10.1016/j.solmat.2021.111055
|
| [14] |
Sa L, Ea T. Synthesis of vandium of vandium oxide powders by evaporative decomposition of solutions[J]. Journal of the American Ceramic Society, 1995,1:104−108.
|
| [15] |
Zhao Zhengjing, Yi Liu, Yu Zhinong, et al. Sn-W Co-doping improves thermochromic performance of VO2 films for smart windows[J]. ACS Applied Energy Materials, 2020,3(10):9972−9979. doi: 10.1021/acsaem.0c01651
|
| [16] |
Chen Zhang, Gao Yanfeng, Kang Litao, et al. Fine crystalline VO2 nanoparticles: synthesis, abnormal phase transition temperatures and excellent optical properties of a derived VO2 nanocomposite foil[J]. Journal of Materials Chemistry, 2014,2:2781. doi: 10.1039/c3ta13727a
|
| [17] |
Li Dengbing, Li Ming, Pan Jing, et al. Hydrothermal synthesis of Mo-doped VO2/TiO2 composite nanocrystals with enhanced thermochromic performance[J]. Acs Applied Materials & Interfaces, 2014,6(9):6555−6561.
|
| [18] |
Huang Weigang, Lin Hua, Tu Mingjing. Preparation of VO2 nanopowder by thermal decomposition of VOC2O4·H2O and its phase transition characteristic[J]. Journal of Functional Materials, 2006,3:440−441. (黄维刚, 林华, 涂铭旌. VOC2O4·H2O热分解制备纳米VO2粉体及相变特性[J]. 功能材料, 2006,3:440−441. doi: 10.3321/j.issn:1001-9731.2006.03.031
|
| [19] |
Peng Zifei, Wei Jiang, Liu Heng. Synthesis and electrical properties of tungsten-doped vanadium dioxide nanopowders by thermolysis[J]. The Journal of Physical Chemistry C, 2007,111:1119−1122.
|
| [20] |
Kong Fongyu, Li Ming, Pan Shusheng, et al. Synthesis and thermal stability of W-doped VO2 nanocrystals[J]. Materials Research Bulletin ,2011, 46: 2100-2104.
|
| [21] |
Lin Hua, Zou Jian, Li Qing. Preparation and characterization of VO2 nano-powder by thermal decomposing VOC2O4·H2O[J]. Iron Steel Vanadium Titanium, 2006,21(1):55−58. (林华, 邹建, 李庆. 草酸氧钒热分解制备纳米VO2及粉体表征[J]. 钢铁钒钛, 2006,21(1):55−58.
|




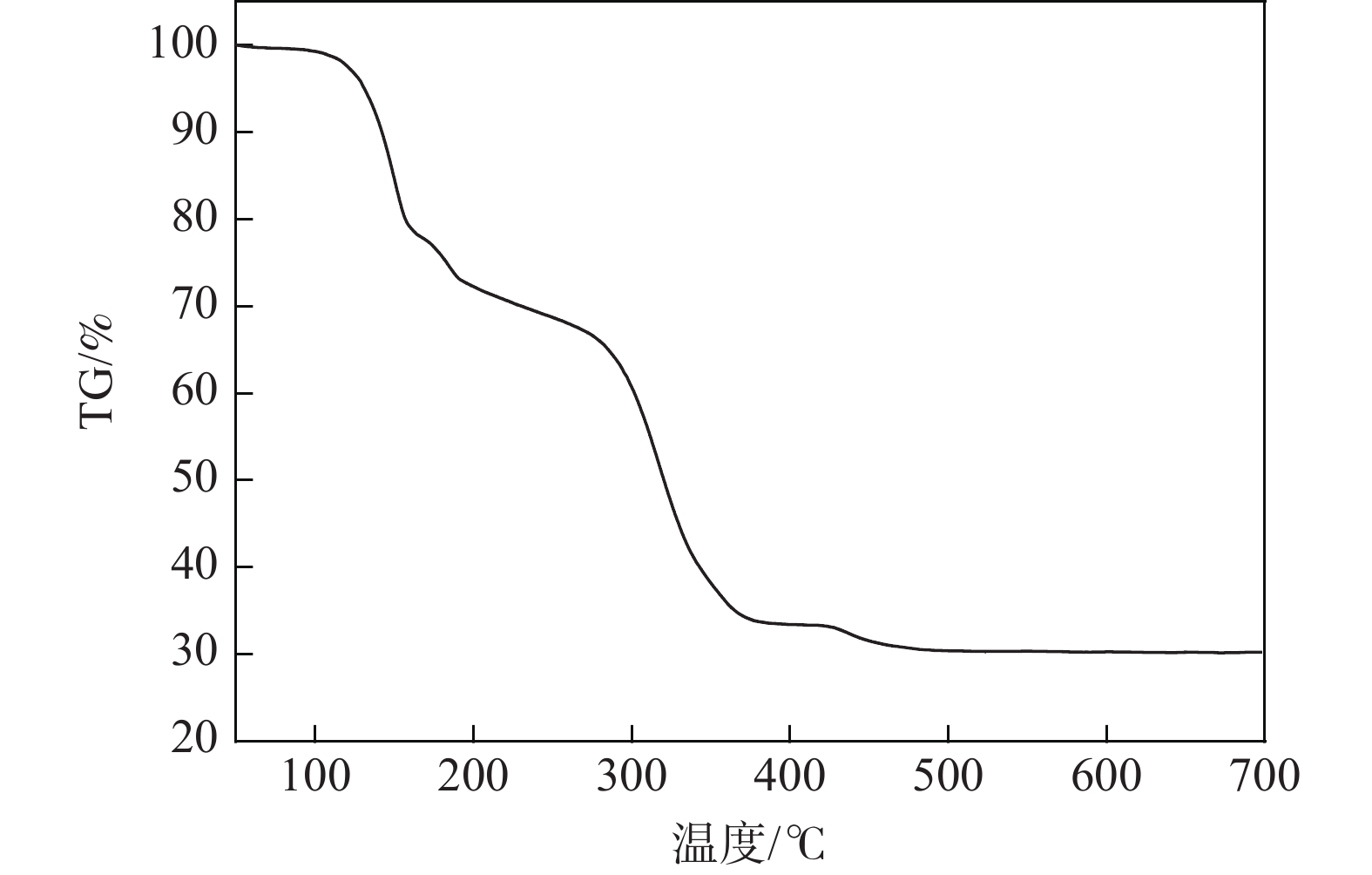
 下载:
下载: