Experimental study on the acidolysis reaction of low-grade titanium slag
-
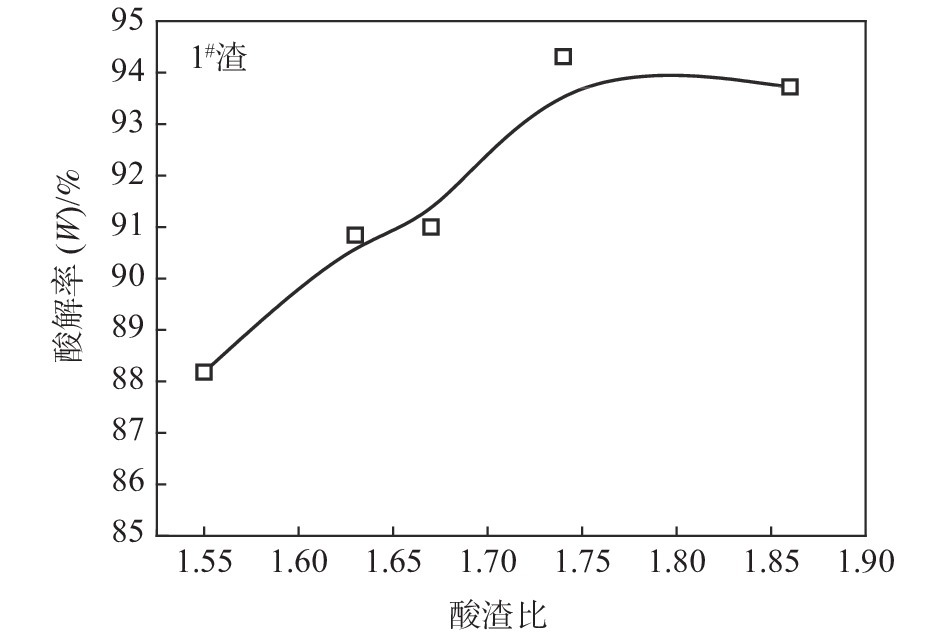
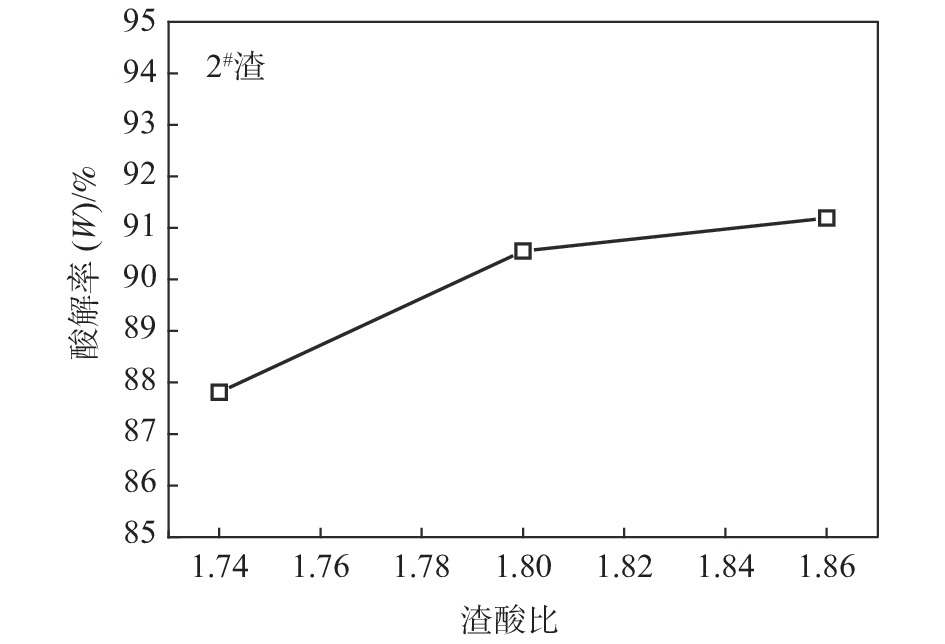
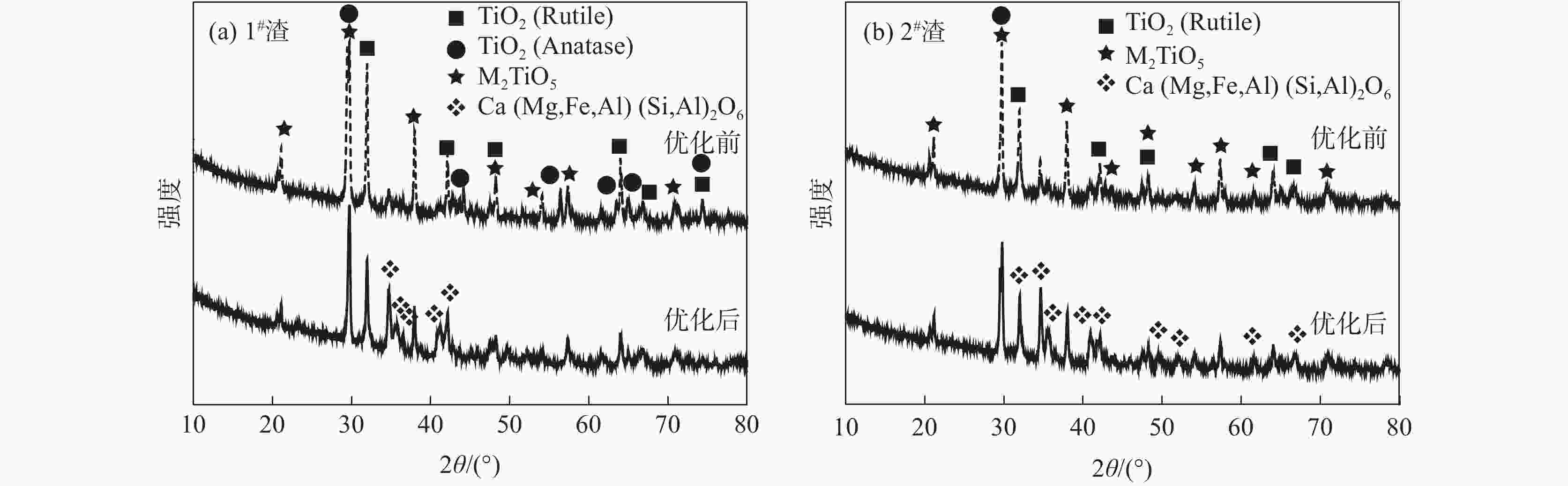
摘要: 针对低品位钛渣酸解率低的问题,研究了低品位钛渣和74渣的物相组成、钛渣酸解温度曲线和化学反应标准生成热之间的差异,考察了反应酸浓度、引发温度、主反应时间、酸渣比对低品位钛渣酸解率的影响。结果表明:低品位钛渣酸解率低不是由难溶于硫酸的物相引起,而是与酸解反应速率有关;引发温度高引起酸解反应速率快、主反应时间短是低品位钛渣酸解率低的主要原因之一;适当降低引发温度,可以满足低品位钛渣酸解需要外加热补充热量需求;通过降低反应酸浓度和引发温度,延长主反应时间,1#渣的酸解率由78.98%提高至94.31%,2#渣的酸解率由71.77%提高至91.19%;与优化前相比,酸解工艺优化后残渣物相中黑钛石相(M2TiO5)峰值减弱,且新增了辉石相(Ca(Mg,Fe,Al)(Si,Al)2O6)。Abstract: Regarding the problem that low acidolysis rate occurs frequently at the beginning of the experiment in low-grade titanium slag, an investigation was proposed to elaborate the relationship among the phase composition, acidolysis temperature curve, and standard heat of reaction for the chemical reaction in low-grade titanium slag and slag 74 (the titanium slag is 74%). Moreover, the effects of acid concentration, initiation temperature, primary reaction time, and acid-slag ratio on the acid hydrolysis rate of low-grade titanium slag were also investigated. The results show that the low acidolysis rate in low-grade titanium slag is not caused by the formation of the insoluble sulfuric acid phase but is related to the acidolysis reaction rate. One of the main reasons for the increasing acidolysis reaction rate and the decreasing primary reaction time in low-grade titanium is the increased initiation temperature. Appropriately reducing the initiation temperature can meet the demand of external heating to supplement heat for acidolysis in low-grade titanium slag. The acidolysis rate of slag 1# increases from 78.98% to 94.31%, and that of slag 2# increases from 71.77% to 91.19%, respectively, by reducing the concentration of reaction acid and the initiation temperature and prolonging the primary reaction time. In addition, compared with the phase of the residue obtained before optimization, the peak value about M2TiO5 is decreased, and a new phase (Ca(Mg,Fe,Al)(Si,Al)2O6) is formed in the phase of the residue.
-
表 1 钛渣主要化学成分
Table 1. Main chemical compositions of titanium slag
% 编号 TiO2 FeO MgO Al2O3 CaO SiO2 1#渣 63.95 21.61 6.24 1.99 1.22 4.12 2#渣 68.33 12.99 8.51 2.47 1.52 4.59 3#渣 74.86 8.88 7.06 2.53 1.66 5.54 表 2 不同品位钛渣物相组成
Table 2. Phase composition of titanium slag in different grades
% 编号 黑钛石 黑钛石
+钛辉石钛辉石 金属铁 金属钛(铁) 硫化铁 钛铁矿 金红石 1#渣 77.64 1.39 12.14 1.09 6.68 0.16 0.73 2#渣 81.25 1.06 11.09 3.36 2.41 0.11 0.22 0.07 3#渣 80.29 13.72 0.65 0.69 0.11 2.17 表 3 低品位钛渣和74渣酸解反应放热量
Table 3. Heat releases of acidolysis reactions in low-grade titanium slag and slag 74
kJ 编号 二氧化钛与硫酸
反应放热氧化亚铁与硫酸
反应放热氧化镁与硫酸
反应放热氧化铝与硫酸
反应放热氧化钙与硫酸
反应放热酸解反应
总放热1#渣 19.50 36.38 20.33 2.53 5.90 84.64 2#渣 20.84 21.87 27.73 3.14 7.35 80.93 3#渣 22.83 14.95 23.00 3.22 8.03 72.03 表 4 不同反应酸浓度的1#渣酸解率
Table 4. Acidolysis rates of slag 1# in different reaction acid concentrations
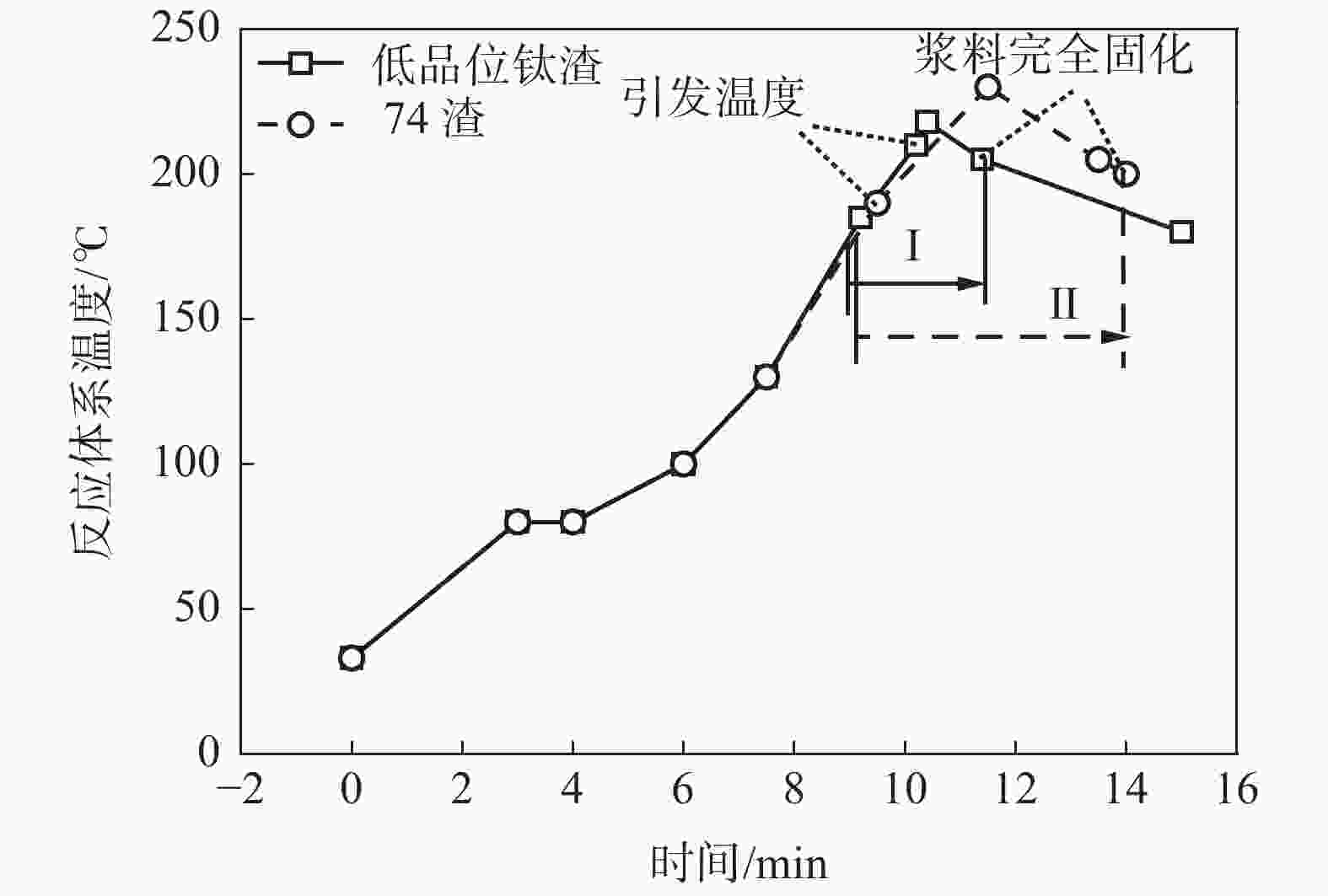
反应酸浓度/% 酸渣比 引发温度/℃ 熟化温度/℃ 酸解率/% 92 1.74∶1 210 175 78.98 89 1.74∶1 210 175 80.80 86 1.74∶1 210 175 84.81 表 5 不同引发温度的1#渣酸解率
Table 5. Acidolysis rates of slag 1# in different initiation temperatures
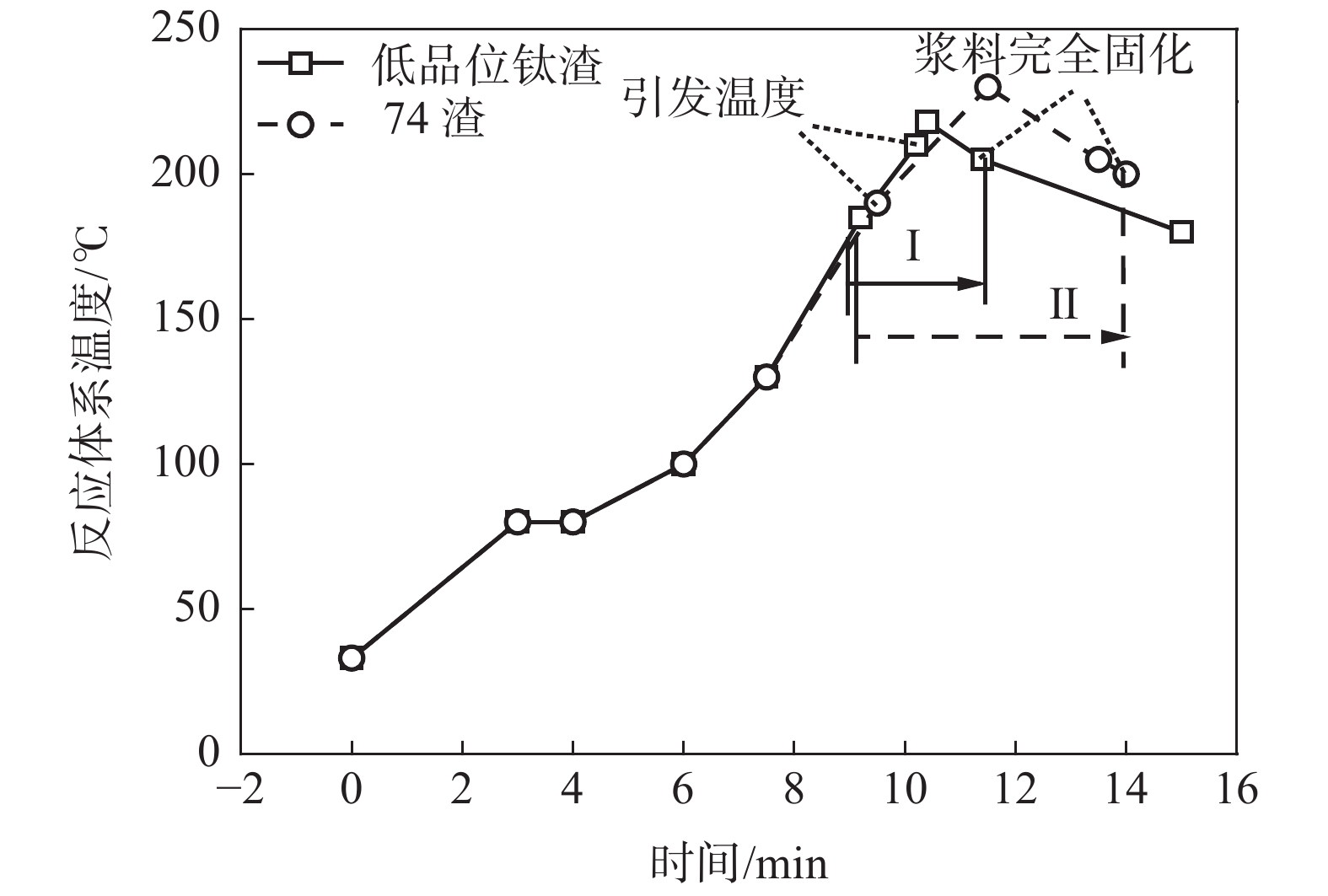
引发温度/℃ 酸渣比 反应体系最高温度/℃ 主反应时间/min 熟化温度/℃ 酸解率/% 210 1.86∶1 218 2.4 175 70.64 135 1.86∶1 177 0.5 175 82.53 185 1.86∶1 203 2.5 175 83.35 注:反应体系温度180 ℃至浆料完全固化时温度区间定义为“主反应时间”。 表 6 不同主反应时间的1#渣酸解率
Table 6. Acidolysis rates of slag 1# in different main reaction times
试验编号 引发温度/
℃酸渣比 反应体系
最高温度/℃主反应时间/
min熟化温度/
℃浆料完全
固化温度/℃开始熟化时
浆料温度/℃酸解率/
%待反应体系温度下降时,
是否继续外部加热SH-1 185 1.86:1 203 2.5 175 205 205 83.35 是 SH-2 185 1.86:1 203 8.0 175 未固化 180 77.45 否 SH-3 185 1.86:1 203 9.0 160 158 158 93.72 否 注:反应体系温度180 ℃至浆料完全固化时温度区间定义为“主反应时间”;由于SH-2开始熟化时浆料未固化,故其主反应时间是指反应体系温度180 ℃至熟化前浆料温度。 表 7 不同引发温度的2#渣酸解率
Table 7. Acidolysis rates of slag 2# in different initiation temperatures
引发温度/℃ 反应酸浓度/% 酸渣比 反应体系最高温度/℃ 主反应时间/min 浆料完全固化温度/℃ 熟化温度/℃ 酸解率/% 200 86 1.74∶1 218 2.5 205 175 73.16 185 86 1.74∶1 209 2.6 200 175 74.19 160 86 1.74∶1 198 7.2 172 175 87.81 注:反应体系温度180 ℃至浆料完全固化时温度区间定义为“主反应时间”。 -
[1] Bi Sheng. Status and development of titanium dioxide industry in 2019[J]. Iron Steel Vanadium Titanium, 2019,40(4):1−3. (毕胜. 2019年中国钛白粉工业状况及发展趋势[J]. 钢铁钒钛, 2019,40(4):1−3. doi: 10.7513/j.issn.1004-7638.2019.04.001 [2] Tang Jian. Analysis on production cost of titanium dioxide by sulfuric acid method[J]. Inorganic Chemicals Industry, 2009,(6):43−46. (唐建. 硫酸法钛白粉生产成本分析[J]. 无机盐工业, 2009,(6):43−46. doi: 10.3969/j.issn.1006-4990.2009.06.014 [3] Xiao Jun. A brief analysis of the industrial development of titanium dioxide by sulfuric acid method and Panzhihua titanium slag[J]. China Nonferrous Metallurgy, 2019,(5):31−35. (肖军. 硫酸法钛白与攀枝花钛渣产业发展浅析[J]. 中国有色冶金, 2019,(5):31−35. doi: 10.3969/j.issn.1672-6103.2019.05.009 [4] Zhang Shuli, Cheng Xiaozhe, Hu Hongfei. Study on digestion technology of acid-dissolved titanium slag[J]. Iron Steel Vanadium Titanium, 2003,24(1):39−45. (张树立, 程晓哲, 胡鸿飞. 酸溶性钛渣酸解工艺研究[J]. 钢铁钒钛, 2003,24(1):39−45. doi: 10.3969/j.issn.1004-7638.2003.01.008 [5] Zou Jianxin, Wang Rongkai. Study on acid digesting titania slag during titanium dioxide production[J]. Modern Chemical Industry, 2003,26:273−276. (邹建新, 王荣凯. 钛渣替代钛矿制备钛白酸解试验研究[J]. 现代化工, 2003,26:273−276. [6] Wang Bin, Cheng Xiaozhe, Han Kexi, et al. Research of acidolysis performance of acid-soluble titanium slag[J]. Iron Steel Vanadium Titanium, 2009,30(2):6−11. (王斌, 程晓哲, 韩可喜, 等. 酸溶性钛渣酸解性能研究[J]. 钢铁钒钛, 2009,30(2):6−11. doi: 10.7513/j.issn.1004-7638.2009.02.002 [7] Shui Bigang, Ma Weiping, Cheng Xiaozhe, et al. Study on continuous acidolysis technology of titanium slag[J]. Inorganic Chemicals Industry, 2013,45(9):31−34. (税必刚, 马维平, 程晓哲, 等. 钛渣连续酸解工艺技术研究[J]. 无机盐工业, 2013,45(9):31−34. doi: 10.3969/j.issn.1006-4990.2013.09.010 [8] Li Liang. Research on the acidolysis technology of Panzhihua vanadic titanomagnetite deeply reducing slag[J]. Light Metals, 2010,(5):50−55. (李亮. 熔分渣酸解性能研究[J]. 轻金属, 2010,(5):50−55. -




 下载:
下载: