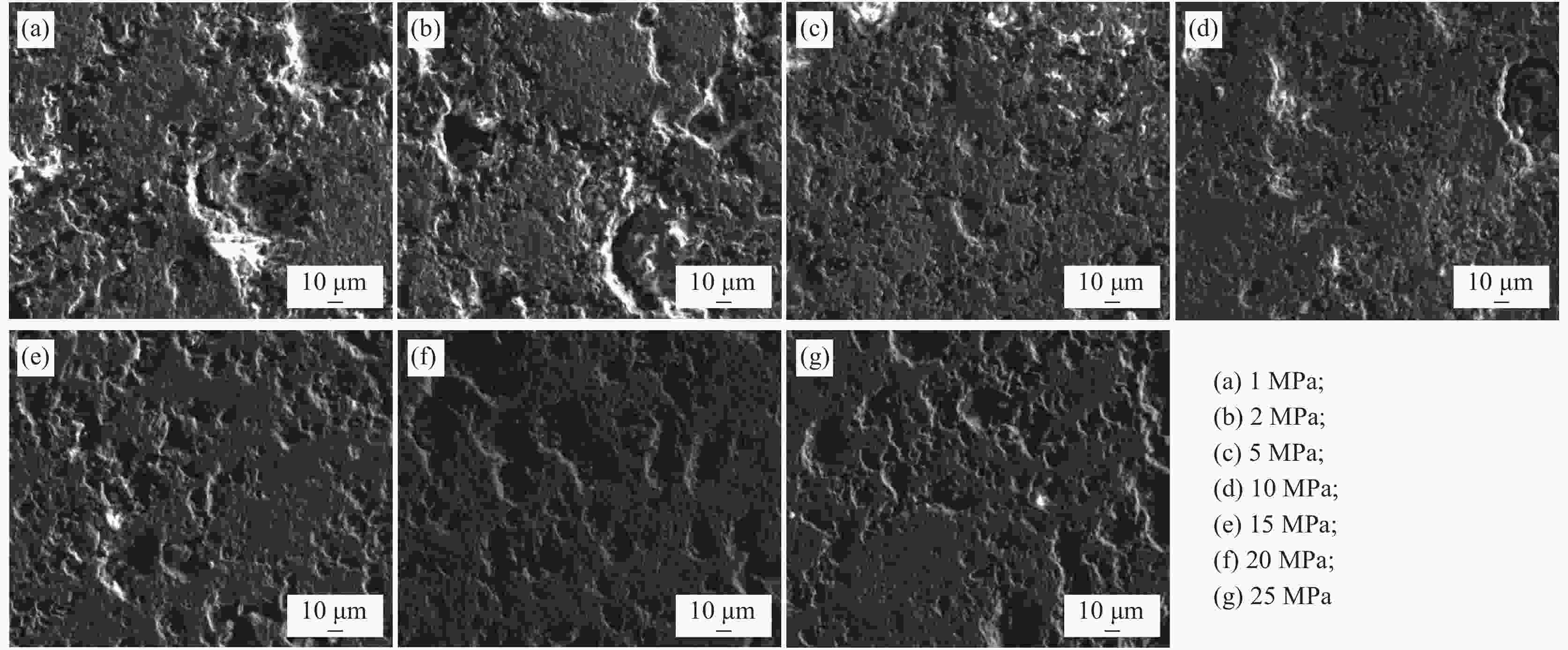
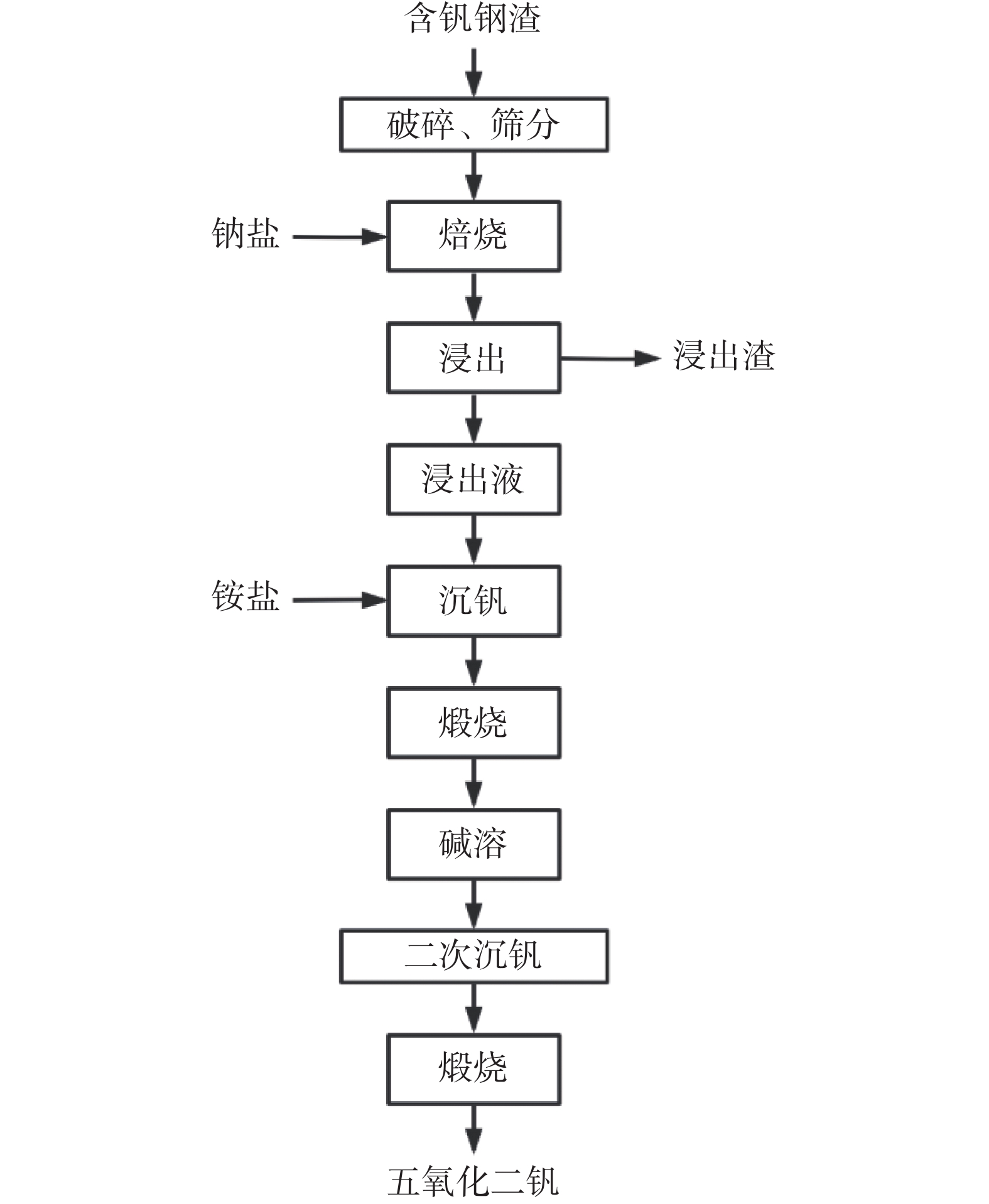
Exploration of roasting vanadium extraction from vanadium slag and Na2O2
-
摘要: 将钒渣与过氧化钠(Na2O2)混和,经压块、焙烧、水浸等处理工艺,将钒富集到V2O5中。研究了焙烧温度、压块压力、焙烧时间、Na2O2加入量、浸出温度等因素对钒浸出率的影响。结果表明,当Na2O2与钒渣中V2O5的摩尔比在0.5∶1~4∶1、焙烧温度在700~1000 ℃、压块压力在1~25 MPa范围内变化时,钒浸出率均呈现先增大后减小的规律,且当钠钒比(Na2O2/V2O5)为3∶1、焙烧温度为850 ℃、压块压力为5 MPa时钒浸出率最大,为95.57%。焙烧时间在0.5~2.5 h、浸出温度在60~100 ℃时,钒浸出率出现波动,在焙烧时间为2.5 h、浸出温度为80 ℃时达到最大值。最优试验条件下,钒浸出率为95.57%。同时,使用X射线衍射分析仪和电子探针分析和表征了焙烧熟料中的物相及其分布规律。结果表明,焙烧后熟料中主要的物相有Fe2O3,Fe3O4,Ca(TiO3),Na3VO4,Mg0.165Mn0.835O等。最后,将熟料加入铵盐再经过沉淀和煅烧处理得到质量分数为96.84%的V2O5。Abstract: Vanadium slag and sodium peroxide (Na2O2) were mixed, briquetted, roasted, and leached to rich vanadium into V2O5. The effects of roasting temperature, briquetting pressure, roasting time, Na2O2 content, and leaching temperature on the vanadium leaching rate were studied. The results showed that the vanadium leaching rate first increased and then decreased as the molar ratio of Na2O2 to V2O5 increased from 0.5∶1 to 4∶1, the roasting temperature increased from 700 ℃ to 1000 ℃, and the briquetting pressure increased from 1 MPa to 25 MPa. The vanadium leaching rate reached the maximum 95.57% with the Na2O2/V2O5 of 3∶1, the roasting temperature of 850 ℃, and the pressure of 5 MPa. As for the roasting time in the range of 0.5–2.5 h and the leaching temperature of 60–100 ℃, the vanadium leaching rate fluctuated and reached the maximum at 2.5 h and 80 ℃. Under the optimum conditions, the vanadium leaching rate reached 95.57%. Meanwhile, the phases during roasting were analyzed and characterized by X-ray diffraction and electron probe microanalysis. The results showed that the main phases were Fe2O3, Fe3O4, Ca(TiO3), Na3VO4, Mg0.165Mn0.835O. Finally, ammonium salt was added into the leaching solution containing vanadium, and 96.84% V2O5 was obtained through precipitation and calcining process.
-
Key words:
- extraction of vanadium /
- vanadium slag /
- sodiumization roasting /
- Na2O2 /
- briquetting /
- leaching rate
-
表 1 钒渣的主要化学成分
Table 1. Main chemical compositions of V-slag
% Fe2O3 SiO2 MnO TiO2 V2O5 MgO Al2O3 CaO Cr2O3 43.49 14.62 10.84 10.27 8.387 3.00 2.68 2.58 2.41 表 2 V-slag的XRF检测成分
Table 2. XRF detection components of V-slag
% Fe2O3 SiO2 V2O5 Al2O3 CaO P2O5 0.3559 2.5780 96.8448 0.1419 0.0453 0.0342 -
[1] 杨守志. 钒冶金[M]. 北京: 冶金工业出版社, 2010.Yang Shouzhi. Vanadium metallurgy[M]. Beijing: Metallurgical Industry Press, 2010. [2] Peng Kebo, Gao Likun, Rao Bing, et al. Current status of vanadium resources and research progress on vanadium extraction with organic phosphorus extractants[J]. Chinese Journal of Engineering, 2021,43(5):603−611. (彭科波, 高利坤, 饶兵, 等. 钒资源现状及有机磷类萃取剂萃钒的研究进展[J]. 工程科学学报, 2021,43(5):603−611. [3] Moskalyk R R, Aifantazi A M. Processing of vanadium: A review[J]. Minerals Engineering, 2003,16(9):793−805. [4] Xu Zhengzheng, Liang Jinglong, Li Hui, et al. Research status and prospects of vanadium recovery in vanadium containing wastes[J]. Multipurpose Utilization of Mineral Resources, 2020,(3):8−13. (徐正震, 梁精龙, 李慧, 等. 含钒废弃物中钒的回收研究现状及展望[J]. 矿产综合利用, 2020,(3):8−13. doi: 10.3969/j.issn.1000-6532.2020.03.002 [5] Fan Liang, Zhang Wei. Vanadium resources and its preparation technology[J]. Advanced Materials Industry, 2016,(1):41−46. (范亮, 张炜. 钒资源及其制备技术[J]. 新材料产业, 2016,(1):41−46. doi: 10.3969/j.issn.1008-892X.2016.01.010 [6] Hong Ying, Guo Shuanghua, Li Yu, et al. Research progress on extraction technology for vanadium[J]. Guangzhou Chemical Industry, 2021,49(17):23−25. (洪颖, 郭双华, 李雨, 等. 提钒技术研究进展[J]. 广州化工, 2021,49(17):23−25. doi: 10.3969/j.issn.1001-9677.2021.17.008 [7] Zhong Jing, Liu Guobao. Analysis of patent technology of vanadium metallurgical extraction[J]. China Science and Technology Information, 2021,(20):15−16. (钟婧, 刘国宝. 钒冶金提取专利技术分析[J]. 中国科技信息, 2021,(20):15−16. [8] Zhu X, Li W, Zhang C. Extraction and removal of vanadium by adsorption with resin 201*7 from vanadium waste liquid[J]. Environ Res, 2020,180:108865. doi: 10.1016/j.envres.2019.108865 [9] Teng A J, Xue X X. A novel roasting process to extract vanadium and chromium from high chromium vanadium slag using a NaOH-NaNO3 binary system[J]. Journal of Hazardous Materials, 2019,379:120805.1−120805.10. [10] Xie Yu, Ye Guohua, Zuo Qi, et al. New technology of vanadium extraction from vanadium-bearing steel slag[J]. Iron Steel Vanadium Titanium, 2019,40(1):69−77. (谢禹, 叶国华, 左琪, 等. 含钒钢渣提钒新工艺研究[J]. 钢铁钒钛, 2019,40(1):69−77. doi: 10.7513/j.issn.1004-7638.2019.01.013 [11] Chen Desheng, Song Bo, Wang Lina, et al. Direct reduction and enhanced reduction of vanadium-bearing titanomagnetite concentrates[J]. Journal of University of Science and Technology Beijing, 2011,(11):1331−1336. (陈德胜, 宋波, 王丽娜, 等. 钒钛磁铁精矿直接还原反应行为及其强化还原研究[J]. 北京科技大学学报, 2011,(11):1331−1336. [12] Chen Shurui, Yang Shaoli, Ma Lan. Research status of vanadium extraction from steel slag containing vanadium[J]. Conservation and Utilization of Mineral Resources, 2019,39(3):69−74. (陈书锐, 杨绍利, 马兰. 含钒钢渣提钒研究现状[J]. 矿产保护与利用, 2019,39(3):69−74. [13] 李尉. 高钒渣钠化焙烧反应行为研究[D]. 沈阳: 东北大学, 2014.Li Wei. Study on reaction behavior of sodium roasting of high vanadium slag[D]. Shenyang: Journal of Northeastern University, 2014. [14] Li X S, Xie B, Wang G E, et al. Oxidation process of low-grade vanadium slag in presence of Na2CO3[J]. Transactions of Nonferrous Metals Society of China, 2011,21:1860. doi: 10.1016/S1003-6326(11)60942-4 [15] Kozlov V A, Demidov A E. Chemical principles of a technology for making pure vanadium pentoxide[J]. Metallurgist, 2000,44(8):428−433. doi: 10.1007/BF02466148 [16] Gao Minglei, Chen Donghui, Li Lanjie, et al. Dissolution behavior of vanadium from vanadium-bearing steel slag in KOH sub-molten salt[J]. The Chinese Journal of Process Engineering, 2011,11(5):761−766. (高明磊, 陈东辉, 李兰杰, 等. 含钒钢渣中钒在KOH亚熔盐介质中溶出行为[J]. 过程工程学报, 2011,11(5):761−766. [17] Sadykhov G B. Oxidation of titanium-vanadium slags with the participation of Na2O and its effect on the behavior of vanadium[J]. Russian Metallurgy (Metally), 2008,(6):449. [18] Cai Yonghong, Zhao Changming, Ning Zhe, et al. Roasting process of vanadium-bearing steel slag in molten NaOH system[J]. Journal of Central South University(Science and Technology), 2018,49(5):1047−1053. (蔡永红, 赵昌明, 宁哲, 等. 含钒钢渣在熔融NaOH体系中的焙烧过程[J]. 中南大学学报(自然科学版), 2018,49(5):1047−1053. doi: 10.11817/j.issn.1672-7207.2018.05.004 [19] Li H Y, Wang C J, Yuan Y H, et al. Magnesiation roasting-acid leaching: A zero-discharge method for vanadium extraction from vanadium slag[J]. Journal of Cleaner Production, 2020,260:121091. doi: 10.1016/j.jclepro.2020.121091 [20] Yan Hong, Wang Shaona, Du Hao, et al. Reaction rules of carbonization-ammonium process producing vanadium oxide[J]. The Chinese Journal of Nonferrous Metals, 2016,26(9):2023. (闫红, 王少娜, 杜浩, 等. 钒酸钙碳化铵化生产钒氧化物的反应规律[J]. 中国有色金属学报, 2016,26(9):2023. [21] Fu Nianxin, Zhang Lin, Liu Wuhan, et al. Mechanism analysis of phase transformation process in calcified roasting of vanadium slags[J]. The Chinese Journal of Nonferrous Metals, 2018,28(2):377−386. (付念新, 张林, 刘武汉, 等. 钒渣钙化焙烧相变过程的机理分析[J]. 中国有色金属学报, 2018,28(2):377−386. [22] Wang Chunqiong, Liu Wuhan, Liu Huiqian, et al. Research on sintering phenomenon during calcination of vanadium slag[J]. Iron Steel Vanadium Titanium, 2013,34(6):6−11. (王春琼, 刘武汉, 刘恢前, 等. 钒渣钙化焙烧烧结现象研究[J]. 钢铁钒钛, 2013,34(6):6−11. doi: 10.7513/j.issn.1004-7638.2013.06.002 [23] Fu Zibi, Jiang Lin, Li Ming, et al. Simultaneous extraction of vanadium and chromium from vanadium-chromium slag by sodium roasting[J]. Iron Steel Vanadium Titanium, 2020,41(4):1−6. (付自碧, 蒋霖, 李明, 等. 钒铬渣钠化焙烧同步提取钒和铬[J]. 钢铁钒钛, 2020,41(4):1−6. -





 下载:
下载: