Effect of P on catalytic performances of V-Mo/Ti denitration catalyst
-
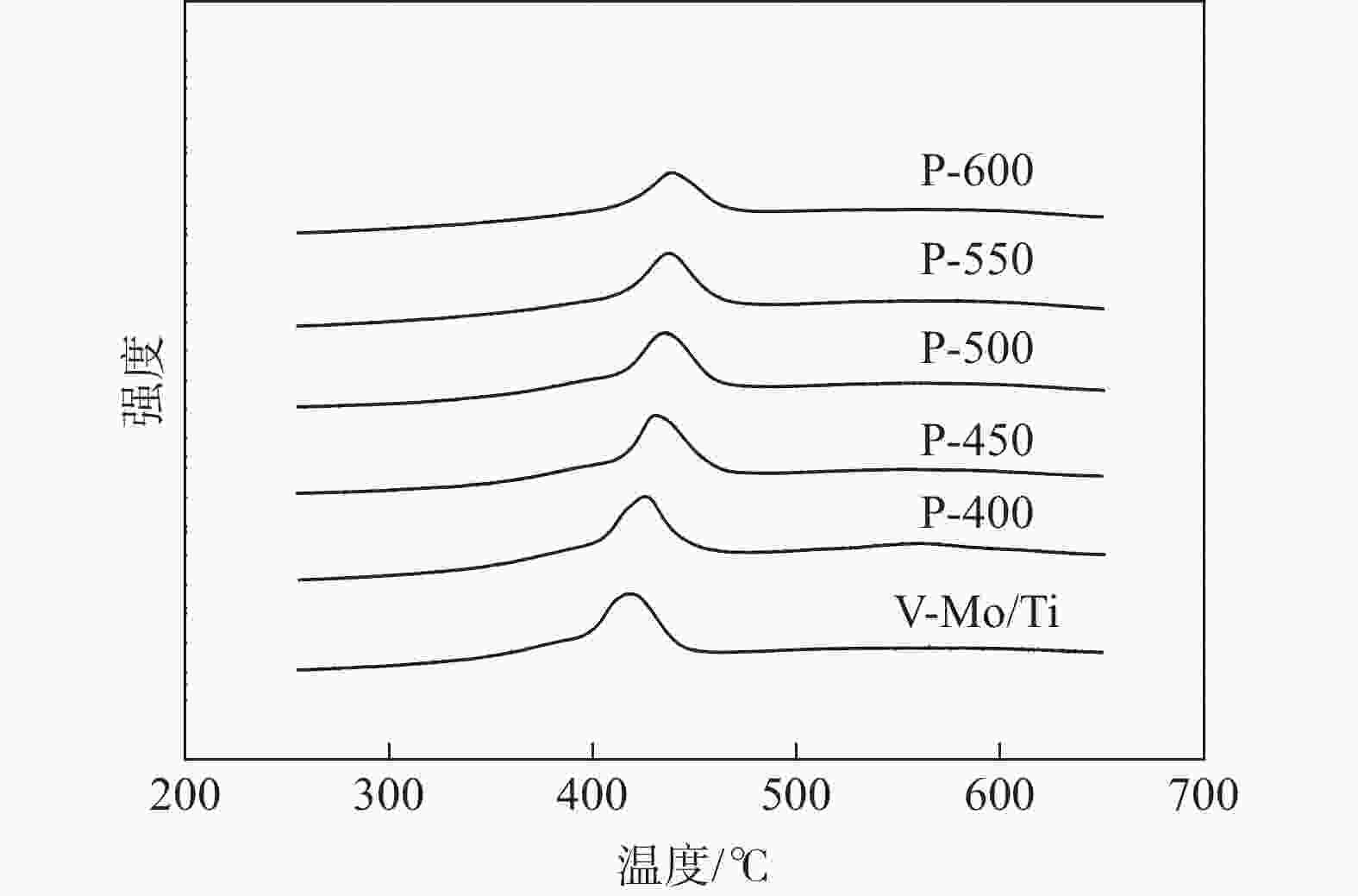
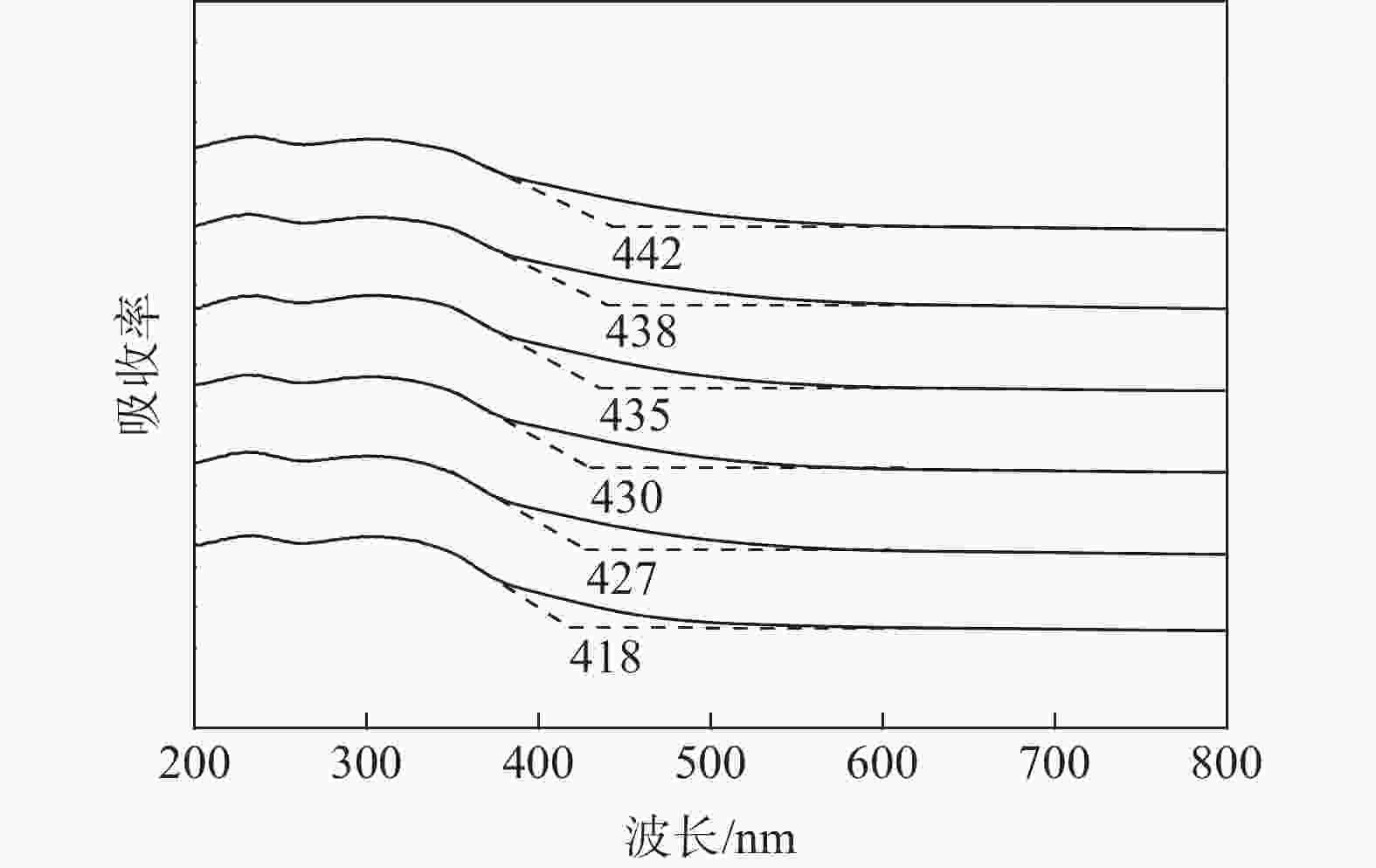
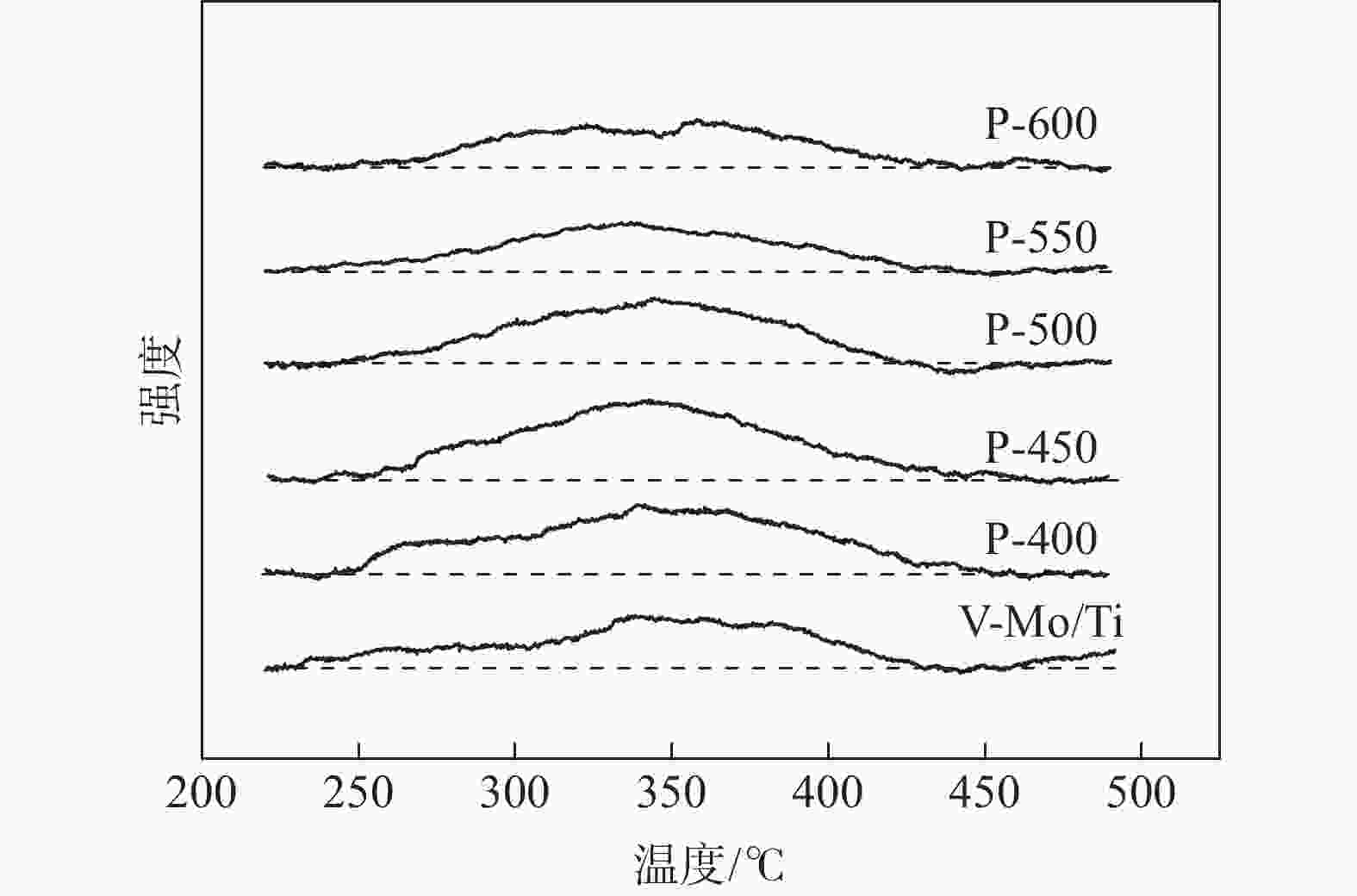
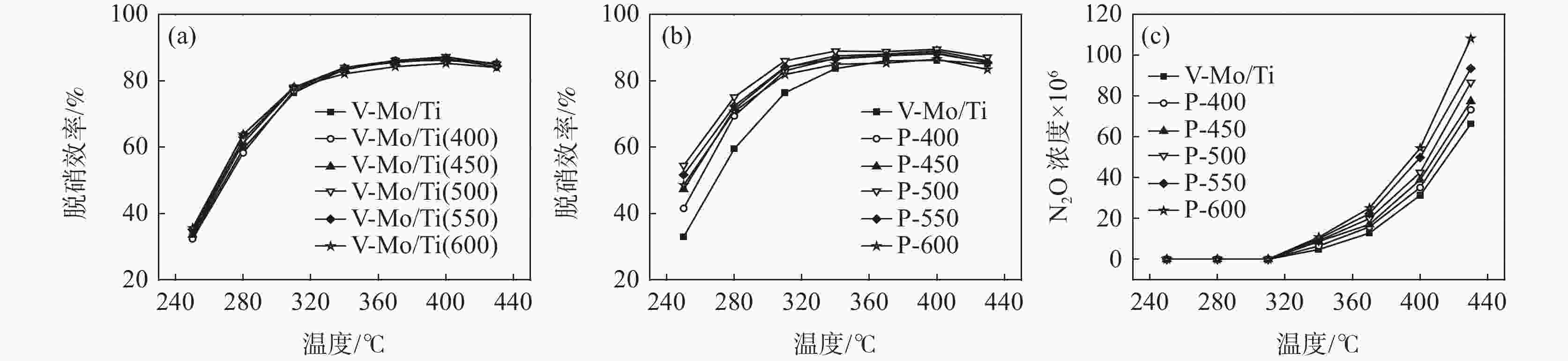
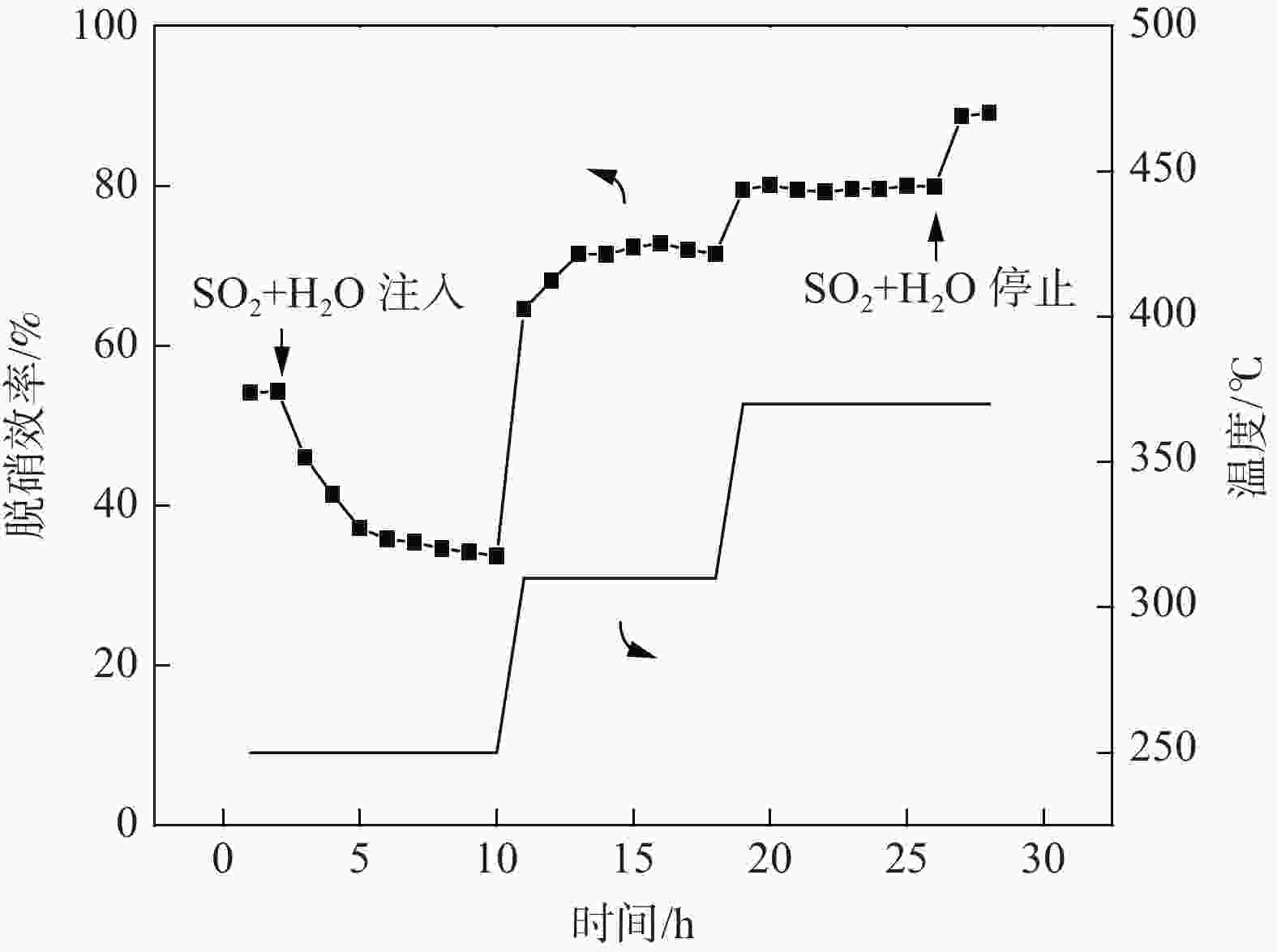
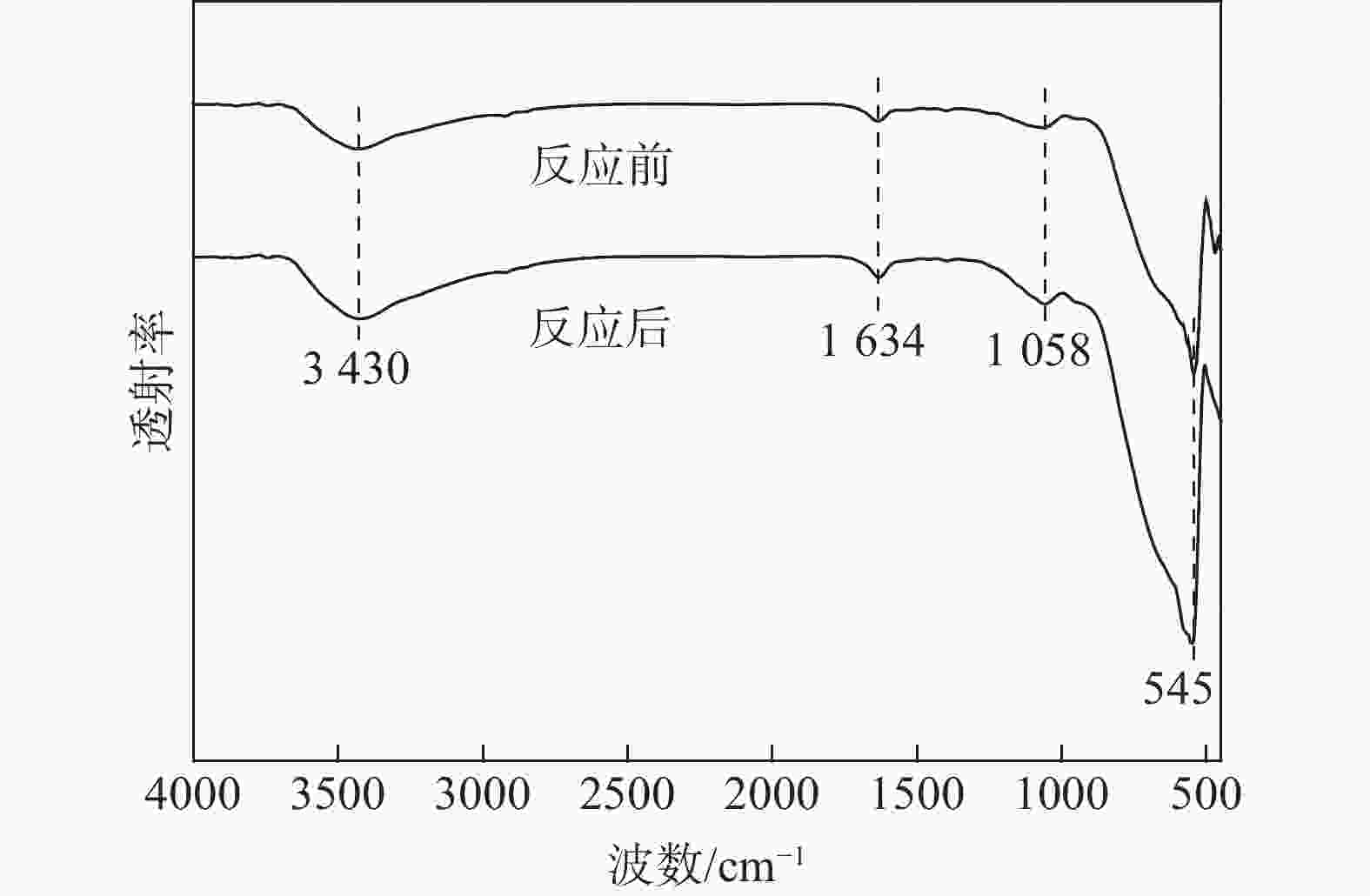
摘要: 为提升工业V-Mo/Ti脱硝催化剂的活性,采用浸渍法对其进行P改性。使用XRD、N2-吸附脱附、XPS、H2-TPR、UV-vis等表征手段对催化剂的物化性质进行分析。随后,在固定床微型反应器上测试了催化剂的脱硝活性。结果显示,向V-Mo/Ti催化剂上负载P后,催化剂的晶型和孔结构无明显变化。P促进了催化剂上聚合钒的生成,导致催化剂(V3++V4+)/V5+比率、化学吸附氧含量的增加。煅烧温度的提升则会进一步促进这个增长趋势,这对催化剂脱硝活性的提升有积极的影响。此外,P的负载还会影响催化剂的酸性。当煅烧温度较低(≤500 ℃)时,V-Mo-P/Ti催化剂的酸量较高。继续升高煅烧温度,催化剂上的P2O5增加,导致其酸性下降。500 ℃煅烧制得的催化剂体现了优良的脱硝活性和抗SO2、H2O性能,具有较好的工业应用前景。
-
关键词:
- V-Mo/Ti催化剂 /
- 脱硝 /
- 改性剂 /
- P /
- 煅烧温度
Abstract: Industrial V-Mo/Ti denitration catalyst was modified by phosphorus (P) via impregnation method to enhance the catalytic performances. The catalyst was characterized by XRD, N2 adsorption and desorption, XPS, H2-TPR and UV-vis. The denitration activities of the catalyst were tested on a fixed-bed micro-reactor. The results show that phosphorus has little impact on the crystallographic form and pore structure of the catalyst. The presence of P could increase the polymeric vanadate of the catalyst, leading to the increase of (V3++V4+)/V5+ ratio and chemisorbed oxygen. This tendency becomes more obvious with the increase of calcination temperature, which is beneficial to the improvement of denitration activities of the catalyst. Besides, the presence of P also affects the acidity of the catalyst. The V-Mo-P/Ti catalyst exhibits relatively higher acidity than that of the V-Mo/Ti catalyst at lower calcination temperatures (≤500 ℃). However, high calcination temperature results in the increase of P2O5, and the acidity of the catalyst decreases accordingly. The catalyst roasted at 500 ℃ exhibits the best catalytic activity and high resistance to SO2 and H2O, possessing a promising perspective for industrial application.-
Key words:
- V-Mo/Ti catalyst /
- denitration /
- modifier /
- phosphorus /
- calcination temperature
-
表 1 不同催化剂的孔结构分析数据
Table 1. Analysis results of pore structure of different catalysts
催化剂 比表面积/(m2·g−1) 孔容/(cm3·g−1) 平均孔径/nm V-Mo/Ti 84.8 0.34 15.2 P-400 82.6 0.32 15.4 P-450 81.4 0.31 15.6 P-500 81.1 0.30 15.7 P-550 80.7 0.28 16.1 P-600 80.1 0.25 16.5 表 2 不同催化剂的XPS分析数据
Table 2. XPS analysis data of different catalysts
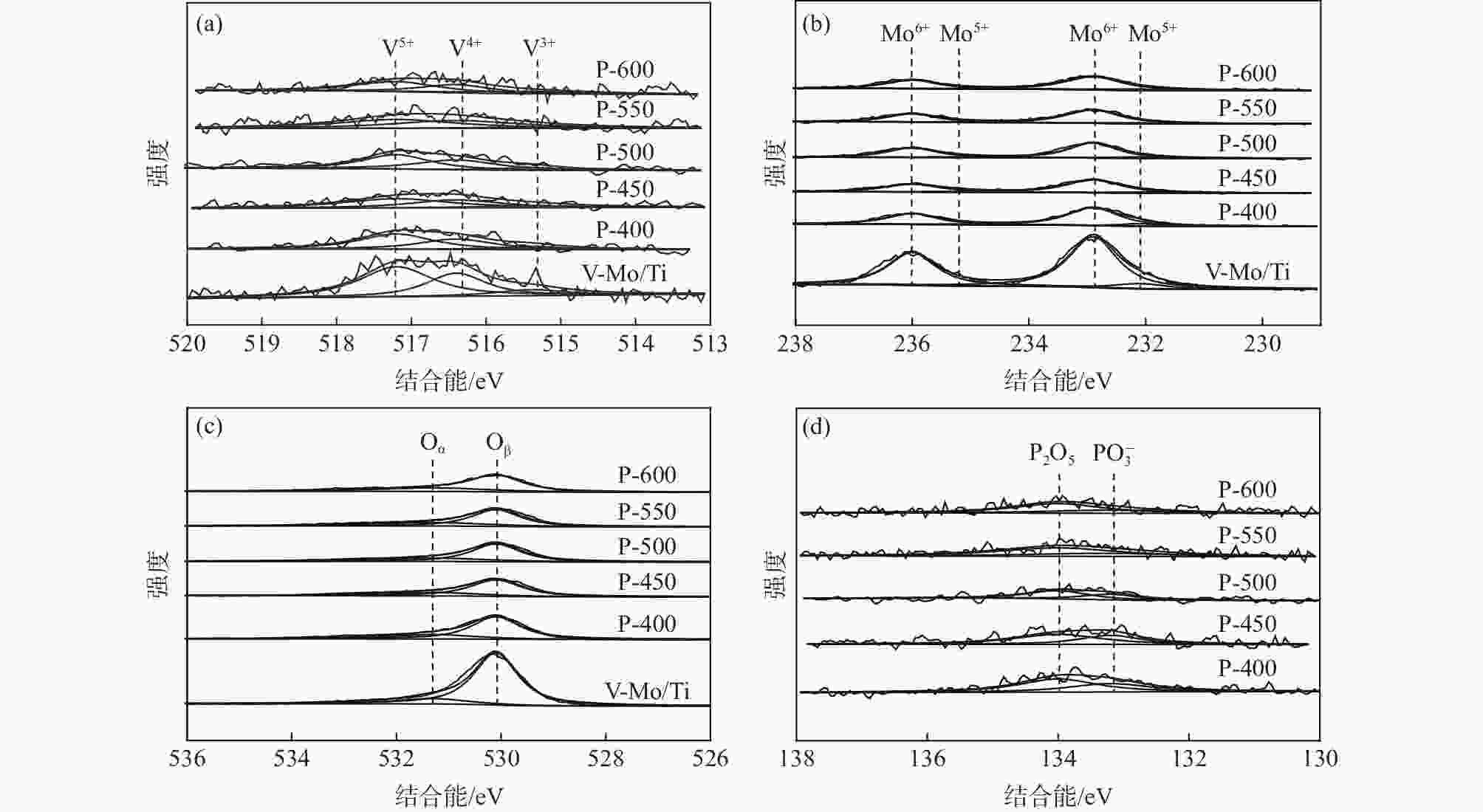
催化剂 (V3++V4+)/V5+ Mo6+/(Mo5++Mo6+) Oα/(Oα+ Oβ) PO3−/(P2O5+PO3−) V-Mo/Ti 0.82 0.93 0.14 P-400 0.86 0.92 0.25 0.40 P-450 0.91 0.91 0.28 0.39 P-500 0.99 0.90 0.31 0.38 P-550 1.02 0.89 0.33 0.33 P-600 1.04 0.88 0.35 0.31 -
[1] Shen Yuesong, Zhu Shemin, Shen Xiaodong. Research process on catalytic materials for selective catalytic reduction of nitrogen oxides[J]. Materials China, 2019,38(12):1125−1134. (沈岳松, 祝社民, 沈晓冬. 选择性催化还原脱硝催化材料研究进展[J]. 中国材料进展, 2019,38(12):1125−1134. [2] Tang Changjin, Sun Jingfang, Dong Lin. Recent process on elimination of NOx from flue gas via SCR technology under ultra-low temperature(<150 ℃)[J]. CIESC Journal, 2020,71(11):4873−4884. (汤常金, 孙敬方, 董林. 超低温(<150 ℃)SCR脱硝技术研究进展[J]. 化工学报, 2020,71(11):4873−4884. [3] Chen Huanzhe, He Haixia, Wan Yameng, et al. Research process of coal-fired flue gas denitrification technology[J]. Applied Chemical Industry, 2019,48(5):1146−1155. (陈欢哲, 何海霞, 万亚萌, 等. 燃煤烟气脱硝技术研究进展[J]. 应用化工, 2019,48(5):1146−1155. doi: 10.3969/j.issn.1671-3206.2019.05.037 [4] Huang Li, Zong Yuhao, Wang Hu, et al. Effect of neodymium addition on the plate-type V2O5-MoO3/TiO2 catalyst for selective catalytic reduction of NO[J]. Rare Metal Materials and Engineering, 2021,50(2):475−482. [5] Chao Jingdi, He Hong, Song Liyun, et al. Promotional effect of Pr-doping on the NH3-SCR activity over the V2O5-MoO3/TiO2 catalyst[J]. Chemical Journal of Chinese Universities, 2015,36(3):523−530. (晁晶迪, 何洪, 宋丽云, 等. Pr掺杂对V2O5-MoO3/TiO2催化剂NH3-SCR反应活性的影响[J]. 高等学校化学学报, 2015,36(3):523−530. [6] 黄力, 王虎, 纵宇浩, 等. Y改性对V2O5-MoO3/TiO2催化剂脱硝性能的影响[J]. 现代化工, 2020, 40(3): 162-166.Huang Li, Wang Hu, Zong Yuhao, et al, Influence of yttrium on denitrification performance of V2O5-MoO3/TiO2 catalyst[J]. Modern Chemical Industry, 2020, 40(3): 162-166. [7] Wu Yanxia, Liang Hailong, Chen Xin, et al. Effect of ZrO2 doping on denitrification performance of V2O5-MoO3/TiO2 catalysts[J]. Environmental Engineering, 2020,38(5):107−119. (吴彦霞, 梁海龙, 陈鑫, 等. ZrO2掺杂对V2O5-MoO3/TiO2催化剂脱硝性能的影响[J]. 环境工程, 2020,38(5):107−119. [8] Wang Jun, Wu Xianghao, Zhou Feixiang, et al. Modification of V-W/TiO2 catalyst for low temperature NH3-SCR on low power load of power plant[J]. Chinese Journal of Environmental Engineering, 2018,12(8):2244−2250. (汪俊, 吴相浩, 周飞翔, 等. 电厂低负荷下V-W/TiO2基NH3-SCR催化剂的低温改性[J]. 环境工程学报, 2018,12(8):2244−2250. doi: 10.12030/j.cjee.201712080 [9] Yan Tao, Liu Qi, Wang Shihao, et al. Promoter rather than inhibitor: Phosphorus incorporation accelerates the activity of V2O5-WO3/TiO2 catalyst for selective catalytic reduction of NOx by NH3[J]. ACS Catalysis, 2020,10(4):2747−2753. doi: 10.1021/acscatal.9b05549 [10] Guo Xiaoyu, Calvin Bartholomew, Willian Hecker, et al. Effects of sulfate species on V2O5/TiO2 SCR catalysts in coal and biomass-fired systems[J]. Applied Catalysis B:Environmental, 2009,92(1−2):30−40. doi: 10.1016/j.apcatb.2009.07.025 [11] Broclawik E, Góra A, Najbar M. The role of tungsten in formation of active sites for no SCR on the V-W-O catalyst surface-Quantum chemical modeling(DFT)[J]. Journal of Molecular Catalysis A:Chemical, 2001,166(1):31−38. doi: 10.1016/S1381-1169(00)00462-3 [12] Al-Kandari H, Al-Kharafi F, Al-Awadia N, et al. The catalytic active sites in partially reduced MoO3 for the hydroisomerization of 1-pentene and n-pentane[J]. Applied Catalysis A:General, 2005,295(1):1−10. doi: 10.1016/j.apcata.2005.07.023 [13] Wang Penglu, Gao Shan, Wang Haiqiang, et al. Enhanced dual resistance to alkali metal and phosphate poisoning: Mo modifying vanadium-titanate nanotubes SCR catalyst[J]. Applied Catalysis A:General, 2018,561(1):68−77. [14] Yao Jia, Liu Shaoguang, Lin Wensong, et al. Study on performance of Ce-Cr-Ni/TiO2 catalysts in CO-SCR[J]. Modern Chemical Industry, 2019,39(5):123−127. (姚佳, 刘少光, 林文松, 等. Ce-Cr-Ni/TiO2催化剂的CO-SCR性能研究[J]. 现代化工, 2019,39(5):123−127. [15] Shawn D Lin, Andreea C Gluhoi, Bernard E Nieuwenhuys. Ammonia oxidation over Au/MOx/γ-Al2O3-activity, selectivity and FTIR measurements[J]. Catalysis Today, 2004,90(1−2):3−14. doi: 10.1016/j.cattod.2004.04.047 [16] Huang Li, Zong Yuhao, Wang Hu, et al. Influence of calcination temperature on the plate-type V2O5-MoO3/TiO2 catalyst for selective catalytic reduction of NO[J]. Reaction Kinetics, Mechanisms and Catalysis, 2018,124(2):603−617. doi: 10.1007/s11144-018-1378-0 [17] Li Mingyuan, Guo Ruitang, Hu Changxing, et al. The enhanced resistance to K deactivation of Ce/TiO2 catalyst for NH3-SCR reaction by the modification with P[J]. Applied Surface Science, 2018,436(1):814−822. [18] Dong Guojun, Zhang Yufeng, Zhao Yuan, et al. Effect of the pH value of precursor solution on the catalytic performance of V2O5-WO3/TiO2 in the low temperature NH3-SCR of NOx[J]. Journal of Fuel Chemistry and Technology, 2014,42(12):1455−1463. doi: 10.1016/S1872-5813(15)60003-2 [19] Tang Fushun, Xu Bolian, Shi Haihua, et al. The poisoning effect of Na+ and Ca2+ ions doped on the V2O5/TiO2 catalysts for selective catalytic reduction of NO by NH3[J]. Applied Catalysis B:Environmental, 2010,94(1-2):71−76. doi: 10.1016/j.apcatb.2009.10.022 [20] Ki Bok Nam, Jong Hyeon Yeo, Sung Chang Hong. Study of the phosphorus deactivation effect and resistance of vanadium-based catalysts[J]. Industrial & Engineering Chemistry Research, 2019,58(41):18930−18941. [21] Li Xiang, Li Junhua, He Xv, et al. Poisoning mechanism and regeneration process of the denitration catalyst[J]. Chemical Industry and Engineering Process, 2015,34(12):4129−4138. (李想, 李俊华, 何煦, 等. 烟气脱硝催化剂中毒机制与再生技术[J]. 化工进展, 2015,34(12):4129−4138. [22] Castellino F, Rasmuss S B, Jense A D, et al. Deactivation of vanadia-based commercial SCR catalysts by polyphosphoric acids[J]. Applied Catalysis B:Environmental, 2008,83(1-2):110−122. doi: 10.1016/j.apcatb.2008.02.008 [23] Gregory T Went, Li-Jen Leu, Richard R Rosin, et al. The effects of structure on the catalytic activity and selectivity of V2O5/TiO2 for the reduction of NO by NH3[J]. Journal of Catalysis, 1992,134(2):492−505. doi: 10.1016/0021-9517(92)90337-H [24] Dong Guojun, Bai Yang, Zhang Yufeng, et al. Effect of the V4+(3+)/V5+ ratio on the denitration activity for V2O5-WO3/TiO2 catalysts[J]. New Journal of Chemistry, 2015,5(39):3588−3596. [25] Juan Antonio Martín, Malcolm Yates, Pedro Ávila, et al. Nitrous oxide formation in low temperature selective catalytic reduction of nitrogen oxides with V2O5/TiO2 catalysts[J]. Applied Catalysis B:Environmental, 2007,70(1):330−334. [26] Yang Jie, Li Xia, Du Hongwei, et al. Preparation and characterization of low-temperature La/Ce doped manganese-based denitration catalyst[J]. Rare Metals and Cemented Carbides, 2021,49(3):41−45. (杨洁, 李侠, 杜红伟, 等. 低温La/Ce掺杂锰基脱硝催化剂的制备及表征[J]. 稀有金属与硬质合金, 2021,49(3):41−45. [27] Jia Yong, Zhou Jun, Bai Jiachuan, et al. Hybrid regeneration of selective catalytic reduction denitration catalyst poisoned by arsenic and potassium[J]. Journal of the Chinese Ceramic Society, 2016,44(7):1025−1032. (贾勇, 周军, 柏家串, 等. 砷、钾复合中毒选择性催化还原脱硝催化剂的再生[J]. 硅酸盐学报, 2016,44(7):1025−1032. [28] Huang Zhanggen, Zhu Zhenping, Liu Zhenyu, et al. Formation and reaction of ammonium sulfate salts on V2O5/AC catalyst during selective catalytic reduction of nitric oxide by ammonia at low temperatures[J]. Journal of Catalysis, 2003,214(2):213−219. doi: 10.1016/S0021-9517(02)00157-4 -





 下载:
下载: