Study on the photocatalytic degradation of disinfection by-product TCM in drinking water by Fe3+/GO-TiO2 thin films
-
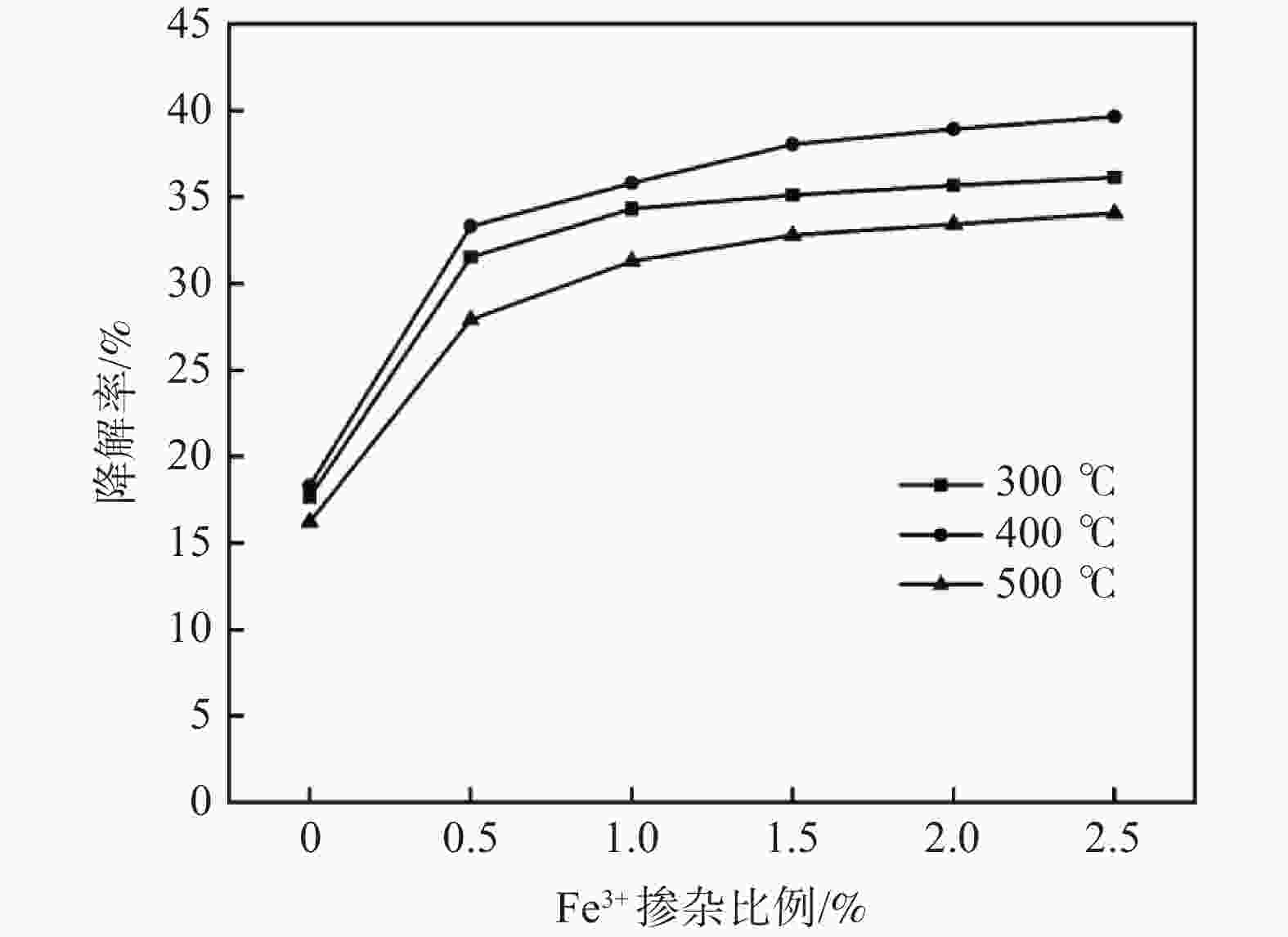
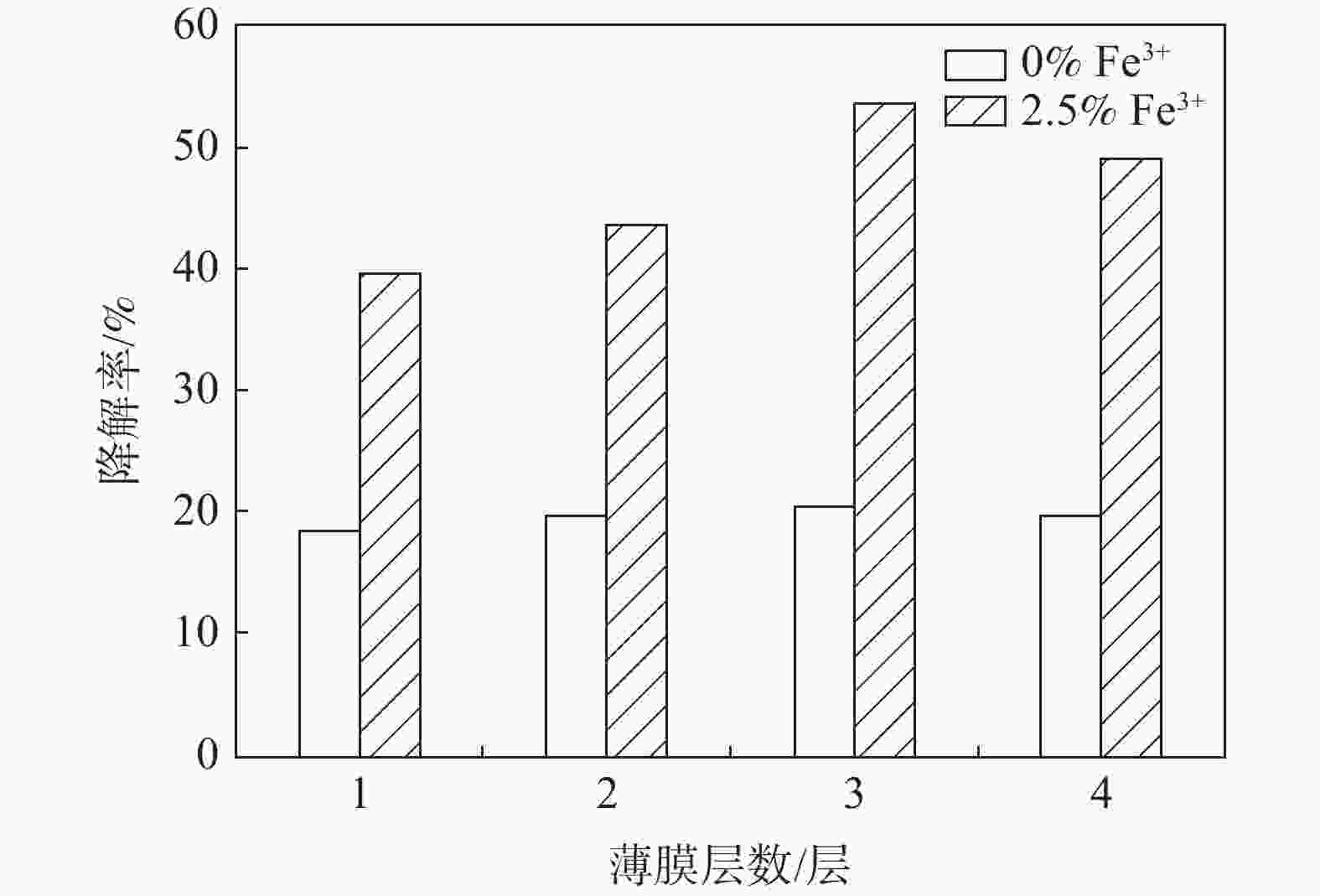
摘要: 通过溶胶凝胶法在玻璃基片上制得不同配比Fe3+掺杂量的GO-TiO2薄膜,对样品采用X-射线衍射和紫外可见光谱分析进行表征。在功率20 W,波长254 nm紫外灯下照射210 min光催化降解三氯甲烷(TCM),考察Fe3+掺杂比例、薄膜焙烧温度、薄膜层数及薄膜复用次数四方面对降解率的影响。结果表明,制得的样品均为锐钛矿型,Fe3+的掺杂使晶体粒径缩小且发生了红移现象。对TCM的降解效果随着Fe3+掺杂比例的增大而提高,400 ℃下焙烧形成的薄膜最佳。无论Fe3+掺杂与否,多次覆膜均能提高TCM降解率。各个薄膜在重复使用四次后降解能力趋于稳定。Abstract: This paper used the sol-gel method to prepare Fe3+ doped GO-TiO2 films with different proportions on glass substrates. The samples were characterized by X-ray diffraction (XRD) and ultraviolet-visible spectrometer (UV-Vis). The photocatalytic degradation of TCM was irradiated under a UV lamp with a power of 20 W and wavelength of 254 nm for 210 min to investigate the effects of Fe3+ doping ratio, film calcination temperature, film layers, and film reuse times on the degradation. The results show that the samples are anatase, and the doping of Fe3+ reduces the crystal size, and a redshift occurs. The degradation effect of TCM increases with the increase of Fe3+ doping ratio, and the film formed by calcination at 400 ℃ is the best. Whether Fe3+ is doped or not, multiple coating can improve the degradation rate of TCM. The degradation ability of each film tends to be stable after being reused four times.
-
Key words:
- modified titanium dioxide /
- sol-gel method /
- photocatalytic performance /
- drinking water /
- TCM /
- degradation rate
-
表 1 掺杂不同比例Fe3+的GO-TiO2粉末的平均晶粒尺寸
Table 1. Average grain sizes of GO-TiO2 samples doped with different ratios of Fe3+
Fe3+掺杂比例/% 平均粒径/nm 0.0 24.5 0.5 13.5 1.0 12.8 1.5 12.5 2.0 12.3 2.5 11.2 表 2 薄膜复用降解TCM效果的对比
Table 2. Effects of reuse of various films on TCM degradation
% Fe3+掺杂比例 首次降解
降解率二次降解
降解率三次降解
降解率四次降解
降解率五次降解
降解率0.0 18.31 14.40 10.42 8.29 8.54 0.5 33.3 29.37 24.2 23.43 21.7 1.0 35.78 30.13 24.75 22.33 21.78 1.5 38.02 34.15 30.08 29.1 28.32 2.0 38.91 35.94 31.73 30.12 28.89 2.5 39.63 36.33 32.8 31.97 31.76 -
[1] Tong Zhongchen, Yuan Jian, Yuan Hongying, et al. Advances in research of trihalomethanes and haloacetic acids of chlorination disinfection by-products in drinking water treatment[J]. Water Purification Technology, 2012,31(2):6−11. (仝重臣, 员建, 苑宏英, 等. 饮用水处理中氯化消毒副产物三卤甲烷和卤代乙酸研究进展[J]. 净水技术, 2012,31(2):6−11. doi: 10.3969/j.issn.1009-0177.2012.02.002 [2] Rook J J. Formation of haloform during chlorination of natural waters[J]. Water Treatm Exam, 1974,23(2):234−243. [3] Bellar T A, Lichtenberg J J, Kroner R C. The occurrence of organohalides in chlorinated drinking waters[J]. Journal American Water Works Association, 1974,66(12):703−706. doi: 10.1002/j.1551-8833.1974.tb02129.x [4] Chen Zhirong, Pei Xinrong, Zhang Fenglan, et al. Research progress and risk assessment of the toxicology of chloroform[J]. Chinese Pharmaceutical Affairs, 2014,28(2):190−194. (陈志蓉, 裴新荣, 张凤兰, 等. 氯仿的毒理学研究现状及氯仿残留的风险性评估[J]. 中国药事, 2014,28(2):190−194. [5] Hu Yanhua, Xu Couyou, Huang Qixiang, et al. Research progress of chlorination disinfection by-products in drinking water on human health and technologies for treatment of drinking water[J]. Guangdong Chemical Industry, 2010,37(4):16−17. (胡衍华, 徐凑友, 黄其祥, 等. 饮用水氯化消毒副产物对人体健康的影响及处理技术研究进展[J]. 广东化工, 2010,37(4):16−17. doi: 10.3969/j.issn.1007-1865.2010.04.008 [6] Han Yaoxia, Zhang Yu, Li Jiaona. Simply analyzing the controlling of the accessory substance from chlorine disinfect in drinking water[J]. Shanxi Architecture, 2006,(1):189−190. (韩耀霞, 张瑜, 李娇娜. 浅析饮用水中氯化消毒副产物的控制[J]. 山西建筑, 2006,(1):189−190. doi: 10.3969/j.issn.1009-6825.2006.01.123 [7] Guo Qing, Zhou Chuanyao, Ma Zhibo, et al. Fundamentals of TiO2 photocatalysis: concepts, mechanisms, and challenges[J]. Advanced Materials, 2019,31(50):1901997. doi: 10.1002/adma.201901997 [8] Zhu Jiaxin, Xiong Yuhua, Guo Rui. Research progress in modification of TiO2 photocatalyst[J]. Inorganic Chemicals Industry, 2020,52(3):23−27,54. (朱佳新, 熊裕华, 郭锐. 二氧化钛光催化剂改性研究进展[J]. 无机盐工业, 2020,52(3):23−27,54. doi: 10.11962/1006-4990.2019-0245 [9] 李宁, 张伟, 李贵贤, 等. TiO2光催化剂的研究进展[J/OL]. 精细化工: 1-11[2021-09-25]. https://doi.org/10.13550/j.jxhg.20210582.Li Ning, Zhang Wei, Li Guixian, et al. Research progress of TiO2 photocatalysts[J/OL]. Fine Chemicals: 1-11[2021-09-25]. https://doi.org/10.13550/j.jxhg.20210582. [10] Wang Xian, Gong Zheng, Yu Qi, et al. Application of modified TiO2 photocatalysis technology in black and odorous water body remediation[J]. Water Purification Technology, 2021,40(9):104−112,118. (汪娴, 龚正, 于琦, 等. TiO2光催化剂改性技术在黑臭水体治理中的应用[J]. 净水技术, 2021,40(9):104−112,118. [11] He Yanan, Jia Ying, Zhang Yongyong, et al. Study of TiO2-graphene oxide by sol-gel method using as a photocatalyst[J]. Applied Chemical Industry, 2015,44(3):506−509,514. (贺亚南, 贾瑛, 张永勇, 等. 溶胶-凝胶法制备TiO2-GO光催化剂的研究[J]. 应用化工, 2015,44(3):506−509,514. [12] 刘美玲. 碳纤维负载二氧化钛光催化材料的制备及其可见光活性研究[D]. 长春: 长春工业大学, 2016.Liu Meiling. Preparation of titania photocatalyst supported on carbon fiber and its photocatalytic activity under visible light[D]. Changchun : Changchun University of Technology, 2016. [13] Zhang Jianwei, Yuan Peng, Wang Jianqiao, et al. Preparation of Ce doped CNTs-TiO2 photocatalyst and its NO oxidation performance[J]. Chinese Journal of Environmental Engineering, 2020,14(7):1852−1861. (张建伟, 苑鹏, 王建桥, 等. Ce掺杂的CNTs-TiO2光催化剂制备及其NO氧化性能[J]. 环境工程学报, 2020,14(7):1852−1861. doi: 10.12030/j.cjee.201909119 [14] 王冀霞. 石墨烯和银掺杂二氧化钛复合膜的光催化性能研究[D]. 秦皇岛: 燕山大学, 2016.Wang Jixia. Study on photocatalytic properties of Ag-TiO2/Graphene composite film[D]. Qinghuangdao: Yanshan University, 2016. [15] GB/T 5750.8—2006. 生活饮用水标准检验方法-有机物指标[S].GB/T 5750.8—2006. Standard examination methods for drinking water-organic parameters[S]. [16] Uwe Holzwarth, Neil Gibson. The scherrer equation versus the 'Debye-Scherrer equation'[J]. Nature Nanotechnology, 2011,6(9):534−534. doi: 10.1038/nnano.2011.145 -





 下载:
下载: