Enhancement of leaching of Ca from steelmaking slag by ultrasonic for CO2 mineral sequestration
-
摘要: 炼钢渣是一种含有大量硅酸钙的碱性废物,可用于钢铁企业捕获CO2并合成高附加值的CaCO3。在此研究了在乙酸溶液中超声对炼钢渣中Ca的浸出率和选择性提取率的影响。试验结果表明,超声功率、液固比和乙酸溶液的初始浓度与Ca的浸出率呈正相关,但炼钢渣的粒度和温度与Ca的浸出率呈负相关。温度和乙酸溶液的初始浓度与Ca的选择性提取率呈负相关。此外,超声功率和固液比对Ca的选择性提取率影响不大,但增加超声功率和固液比会增加非钙杂质元素的浸出率。在0.5 mol/L乙酸水溶液中提取0.96 μm以下炼钢渣中的钙,反应40 min后Ca的选择性提取率高达96.7%。在浸出过程中,超声可以有效打破和去除炼钢渣颗粒表面残留的二氧化硅形成的多孔钝化层,提高Ca的浸出率。Abstract: Steelmaking slags containing large amounts of Ca silicate is a potentially alkaline waste that can be used to capture CO2 to synthesize high-quality CaCO3. Here, effect of ultrasonic on leaching efficiency and selective leaching rate of Ca in steelmaking slags was studied in acetic acid solution. Experimental results shown that ultrasound could help to strengthen leaching of Ca in acetic acid solution. Ultrasonic power, liquid-solid ratio, and initial acetic acid solution concentration were positively correlated with Ca extraction, but particle size of steelmaking slags and temperature were negatively correlated with Ca extraction. Meanwhile, temperature and initial concentration of acetic acid solation had a negative correlation to selective extraction yield of Ca, which were beneficial for the diffusion of impurity elements. Also, ultrasonic power and solid to liquid ratio were insignificant effect to selective extraction yield of Ca, but increase of ultrasonic power and solid to liquid ratio were also helpful to the diffusion of impurity elements. It was worth noting that decreasing grain size of steelmaking slags would raise selective leaching efficiency of Ca and the maximum selective leaching rate upped to 96.7%. During leaching process, ultrasonic could effectively break and remove the porous passivation layer formed by residual silica on surface of steelmaking slags particles, and improved leaching rate of Ca.
-
Key words:
- steelmaking slags /
- CO2 mineral carbonation /
- ultrasonic /
- Ca /
- leaching rate /
- selective leaching rate
-
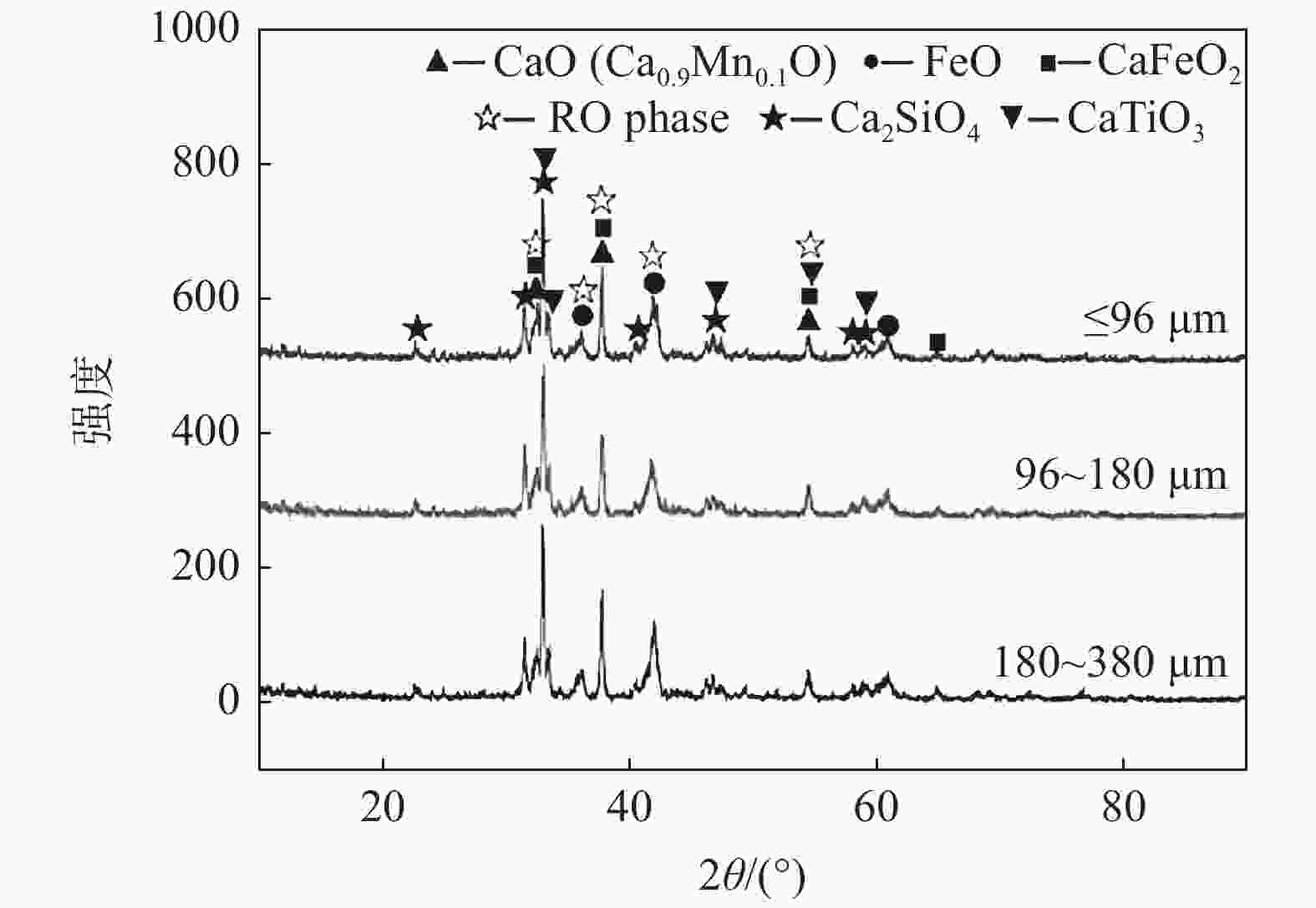
图 5 不同条件浸出渣的SEM形貌
初始乙酸浓度=0.5 mol/L,温度=40 ℃, 时间=40 min(a)原料(粒度=180 ~ 380 μm);(b)机械搅拌(粒度=180 ~ 380 μm);(c)超声(超声功率=100 W, 粒度=180 ~ 380 μm);(d)超声(超声功率=200 W, 粒度=180 ~ 380 μm);(e)超声(超声功率=200 W, 粒度=0 ~ 96 μm);(f)超声(超声功率=200 W, 粒度=96 ~ 180 μm)
Figure 5. SEM morphology of leaching residue under different conditions
表 1 炼钢渣的XRF分析化学成分a
Table 1. Chemical composition of the steelmaking slags by XRF-analysisa
编号 粒度/μm w/% O Ca Fe Si Mg Mn Ti C P V 试样1 180 ~ 380 39.18 28.89 14.33 3.87 3.32 3.18 2.07 1.94 1.12 1.01 试样2 96 ~ 180 37.20 28.76 15.57 3.98 3.78 3.72 1.91 1.70 1.29 1.03 试样3 0 ~ 96 37.57 28.64 15.45 3.93 3.74 3.67 1.92 1.71 1.28 1.00 a只显示含量高于1%的元素。 -
[1] Metz B, Davidson O, Coninck H, et al. Special report on carbon dioxide capture and storage[R]. UK: Cambridge University Press, Cambridge, 2005. [2] Lackner K S. Carbonate chemistry for sequestering fossil carbon[J]. Social Science Electronic Publishing, 2002,27(1):193−232. [3] Huijgen W J J, Comans R N J. Carbon dioxide sequestration by mineral carbonation, literature review[R]. Energy Research Centre of the Netherlands ECN. 2003. [4] Huijgen W J J, Comans R N J. Carbon dioxide sequestration by mineral carbonation: literature review update 2003-2004[R]. Energy Research Centre of the Netherlands ECN. 2005. [5] Wang X, Maroto-Valer M M. Integration of CO2 capture and mineral carbonation by using recyclable ammonium salts[J]. Chem. Sus. Chem., 2011,4(9):1291−1300. doi: 10.1002/cssc.201000441 [6] Werner M, Verduyn M, Mossel G, et al. Direct flue gas CO2 mineralization using activated serpentine: Exploring the reaction kinetics by experiments and population balance modelling[J]. Energy Procedia, 2011,4:2043−2049. doi: 10.1016/j.egypro.2011.02.086 [7] Teir S, Kettle J, Harlin A, et al. Production of silica and calcium carbonate particles from silica minerals for inkjet paper coating and filler purposes[C]//ACEME10. 2010: 63-74. [8] Teir S, Eloneva S, Fogelholm C J, et al. Fixation of carbon dioxide by producing hydromagnesite from serpentinite[J]. Applied Energy, 2009,86(2):214−218. doi: 10.1016/j.apenergy.2008.03.013 [9] Krevor S C, Lackner K S. Enhancing process kinetics for mineral carbon sequestration[J]. Energy Procedia, 2009,1(1):4867−4871. doi: 10.1016/j.egypro.2009.02.315 [10] Zevenhoven R, Teir S, Eloneva S. Heat optimisation of a staged gas–solid mineral carbonation process for long-term CO2 storage[J]. Energy, 2008,33(2):362−370. doi: 10.1016/j.energy.2007.11.005 [11] Boschi C, Dini A, Dallai L, et al. Enhanced CO2 mineral sequestration by cyclic hydraulic fracturing and Si-rich infiltration into serpentinites at Malentrata[J]. Chem. Geol., 2009,265(1-2):209−226. doi: 10.1016/j.chemgeo.2009.03.016 [12] Fagerlund J, Teir S, Nduagu E, et al. Carbonation of magnesium silicate mineral using a pressurised gas/solid process[J]. Energy Procedia, 2009,1(1):4907−4914. doi: 10.1016/j.egypro.2009.02.321 [13] Daval D, Martinez I, Corvisier J, et al. Carbonation of Ca-bearing silicates, the case of wollastonite: experimental investigations and kinetic modelling[J]. Chem. Geol., 2009,265(1-2):63−78. doi: 10.1016/j.chemgeo.2009.01.022 [14] Baldyga J, Henczka M, Sokolnicka K. Utilization of carbon dioxide by chemically accelerated mineral carbonation[J]. Mater. Lett., 2010,64(6):702−704. doi: 10.1016/j.matlet.2009.12.043 [15] Ghoorah M, Dlugogorski B Z, Balucan R D, et al. Selection of acid for weak acid processing of wollastonite for mineralization of CO2[J]. Fuel, 2014,122:277−286. doi: 10.1016/j.fuel.2014.01.015 [16] Machenbach I, Brandvoll O, Wihle J, et al. Development of an industrial process concept for CO2 sequestration by mineral carbonation[C]//Proceedings of the 2nd International Conference on Accelerated Carbonation for Environmental an Materials Engineering. 2008: 459-461. [17] Dufaud F, Martinez I, Shilobreeva S. Experimental study of Mg-rich silicates carbonation at 400 and 500 ℃ and 1 kbar[J]. Chemical Geology, 2009,265(1-2):79−87. doi: 10.1016/j.chemgeo.2009.01.026 [18] Haug T A, Johansen H, Brandvoll O. The way forward for mineral carbonation—importance of collaboration, experiments and modelling[C]// Proceedings of the 3rd International Conference on Accelerated Carbonation for Environmental and Materials Engineering. 2010: 113–119. [19] Zhao H, Li Bao, Wang W, et al. Experimental study of enhanced phosphogypsum carbonation with ammonia under increased CO2 pressure[J]. Journal of CO2 Utilization, 2015,11:10−19. doi: 10.1016/j.jcou.2014.11.004 [20] Said A, Mattila H P, Järvinen M, et al. Production of precipitated calcium carbonate (PCC) from steelmaking slag for fixation of CO2[J]. Applied Energy, 2013,112(4):765−771. [21] Hall C, Large D J, Adderley B, et al. Calcium leaching from waste steelmaking slag: Significance of leachate chemistry and effects on slag grain mineralogy[J]. Minerals Engineering, 2014,65(6):156−162. [22] Revathy T D R, Palanivelu K, Ramachandran A. Direct mineral carbonation of steelmaking slag for CO2, sequestration at room temperature[J]. Environmental Science & Pollution Research, 2016,23(8):7349−7359. [23] Sun Y, Yao M S, Zhang J P, et al. Indirect CO2 mineral sequestration by steelmaking slag with NH4Cl as leaching solution[J]. Chemical Engineering Journal, 2011,173(2):437−445. doi: 10.1016/j.cej.2011.08.002 [24] Gunning P J, Hills C D, Carey P J. Accelerated carbonation treatment of industrial wastes[J]. Waste Management, 2010,30(6):1081−1090. doi: 10.1016/j.wasman.2010.01.005 [25] Chiang Y W, Santos R, Elsen J, et al. Two-way valorization of blast furnace slag into precipitated calcium carbonate and sorbent materials[C]//Proceedings of the 4th International Conference on Accelerated Carbonation for Environmental and Materials Engineering. 2013: 357-367. [26] Nyambura M G, Mugera G W, Felicia P L, et al. Carbonation of brine impacted fractionated coal fly ash: Implications for CO2 sequestration[J]. Journal of Environmental Management, 2011,92(3):655−664. doi: 10.1016/j.jenvman.2010.10.008 [27] Teir S. Fixation of carbon dioxide by producing carbonations from minerals and steelmaking slags[M]. Finland: Espoo, Teknillinen Korkeakoulu, 2008. [28] Uibu M, Uus M, Kuusik R. CO2 mineral sequestration in oil-shale wastes from Estonian power production[J]. Journal of Environmental Management, 2009,90(2):1253−1260. doi: 10.1016/j.jenvman.2008.07.012 [29] Sanna A, Dri M, Hall M R, et al. Waste materials for carbon capture and storage by mineralisation (CCSM) – A UK perspective[J]. Applied Energy, 2012,99(2):545−554. [30] Zhang H N, Xu A J, He D F, et al. Alkaline extraction characteristics of steelmaking slag batch in NH4Cl solution under environmental pressure[J]. Journal of Central South University, 2013,20(6):1482−1489. doi: 10.1007/s11771-013-1638-0 [31] Teir S, Eloneva S, Fogelholm C J, et al. Dissolution of steelmaking slags in acetic acid for precipitated calcium carbonate production[J]. Energy, 2007,32(4):528−539. doi: 10.1016/j.energy.2006.06.023 [32] Hagenson L C, Doraiswamy L K. Comparison of the effects of ultrasound and mechanical agitation on a reacting solid-liquid system[J]. Chemical Engineering Science, 1998,53(1):131−148. doi: 10.1016/S0009-2509(97)00193-0 [33] Santos R M, François D, Mertens G, et al. Ultrasound-intensified mineral carbonation[J]. Applied Thermal Engineering, 2013,57(1-2):154−163. doi: 10.1016/j.applthermaleng.2012.03.035 [34] Sanna A, Lacinska A, Styles M, et al. Silicate rock dissolution by ammonium bisulphate for pH swing mineral CO2 sequestration[J]. Fuel Processing Technology, 2014,120(4):128−135. -
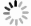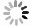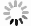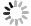



 下载:
下载: