Study on viscosity characteristics of molten vanadium slag during iron-extracting reduction process
-
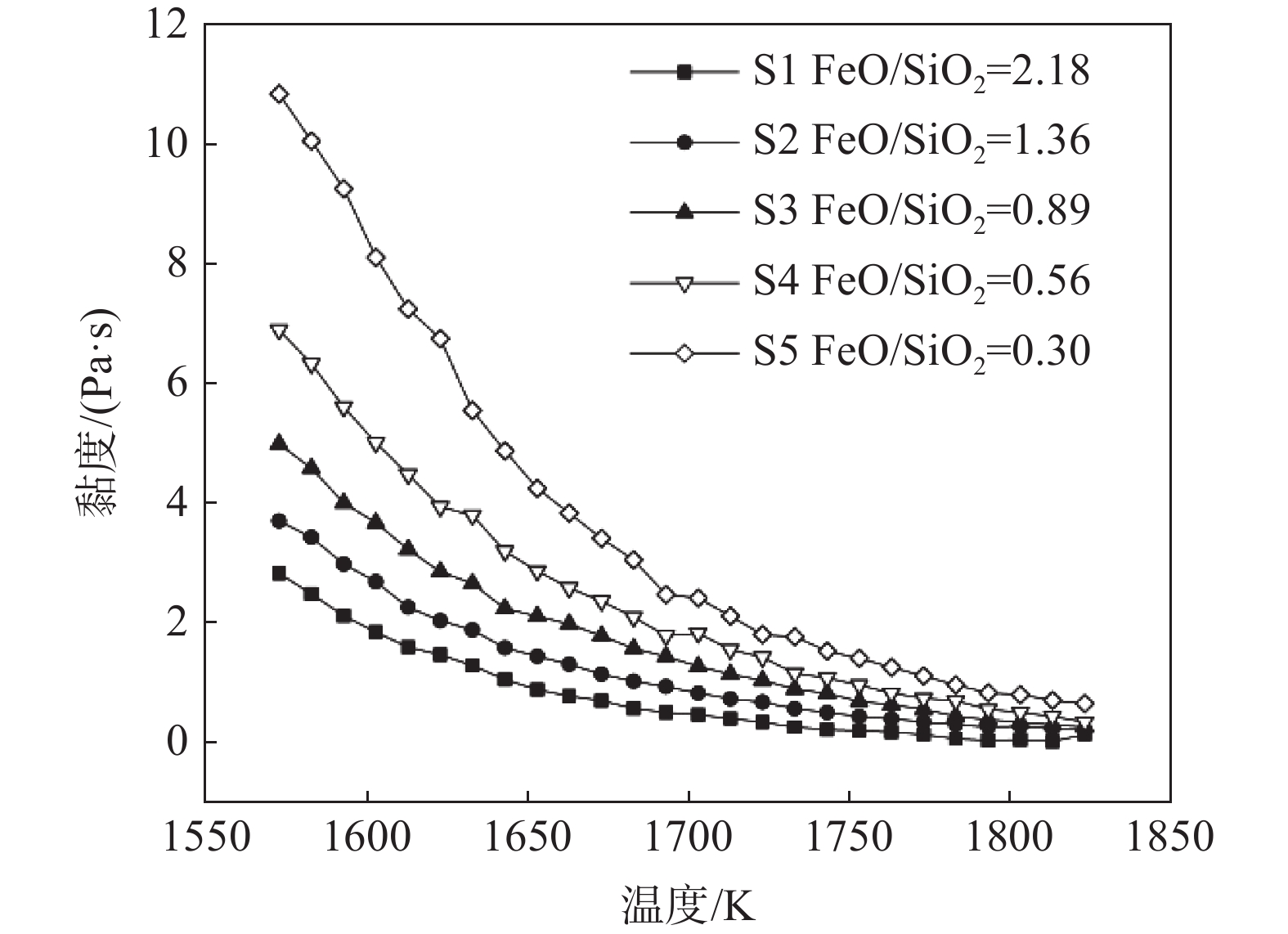
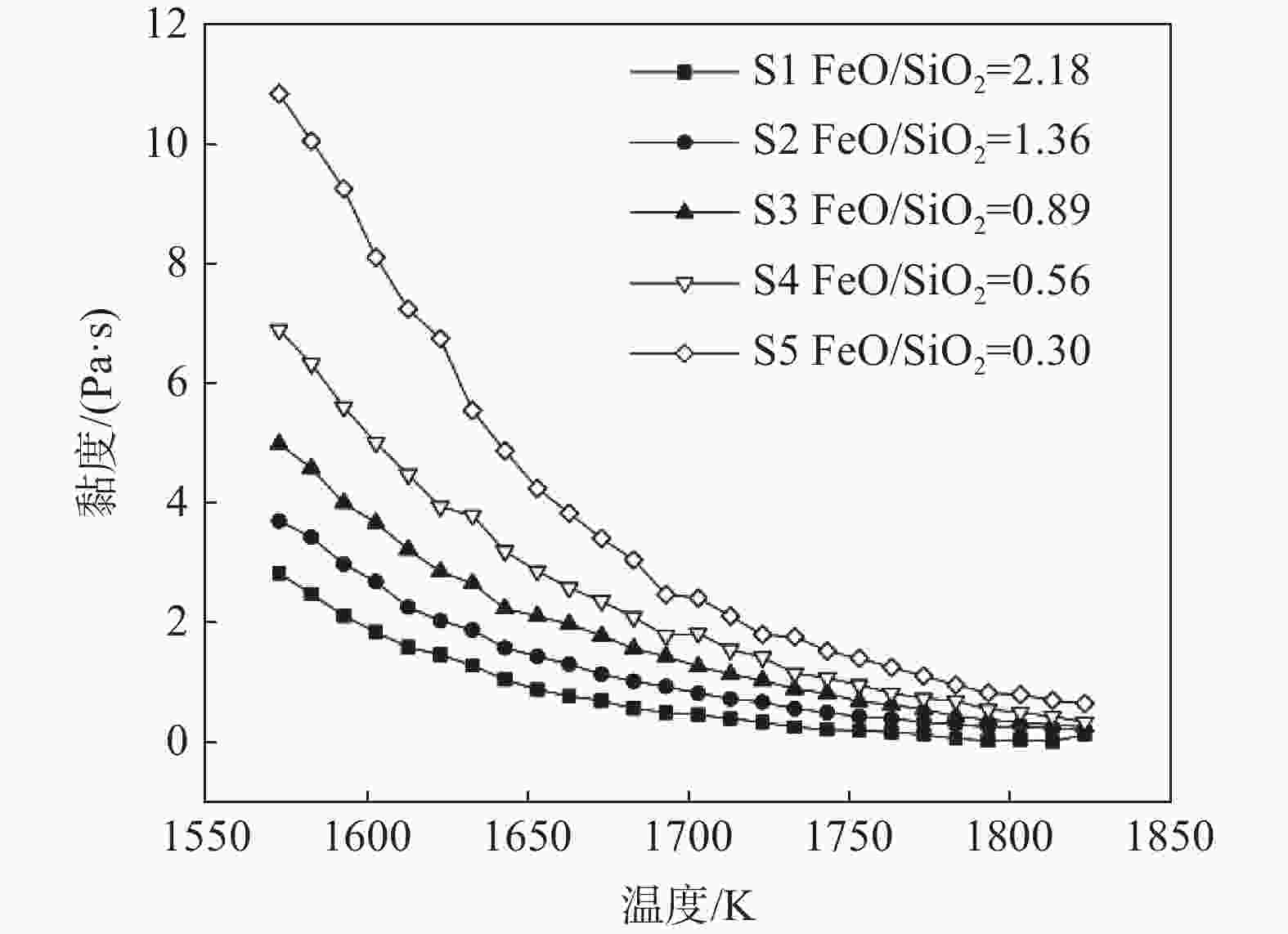
摘要: 研究了在切削废料还原熔融钒渣提铁的工艺进程中熔渣体系黏度的变化规律,并通过对钒渣体系熔体结构的研究,从微观结构角度揭示了熔体中结构单元对宏观黏度的作用机理。结果表明,随还原反应进行,渣中FeO含量不断降低,SiO2含量增加,渣中自由氧离子减少,无法破坏硅酸盐的链、环状结构,导致渣中孤立体、二聚体等简单硅酸盐结构单元减少,链、层状等复杂硅酸盐结构单元增加,熔体聚合度增强,渣系黏度逐渐增加。Abstract: In this paper, the viscosity variation of the vanadium slag system in the iron-extracting reduction process from molten vanadium slag in cutting waste was studied, and the effect mechanism of structural units in the melt on macro-viscosity was revealed from the perspective of microstructure by studying the melt structure of vanadium slag system. Results showed that the content of FeO in the slag decreased, and the content of SiO2 increased during the reduction reaction. Meanwhile, the free oxygen ions in the slag decreased, and the chain and ring silicate structure could not be destroyed, further causing the decrease of simple silicate structural units such as Q0 and Q1, and the increase of complex silicate structural units such as Q2 and Q3. Finally, the melt polymerization degree increased, and the slag viscosity increased gradually.
-
表 1 试验钒渣主要化学成分
Table 1. Main chemical compositions of vanadium residue
% FeO SiO2 V2O3 Cr2O3 MnO TiO2 MgO 40.51 19.04 14.01 4.84 10.97 9.03 1.60 表 2 试验用光伏切削废料的化学成分
Table 2. Chemical composition of photovoltaic cutting waste for testing
% Si SiC SiO2 FeO 56.17 23.56 8.90 11.37 表 3 试验配渣样成分
Table 3. Chemical composition of slagging sample for testing
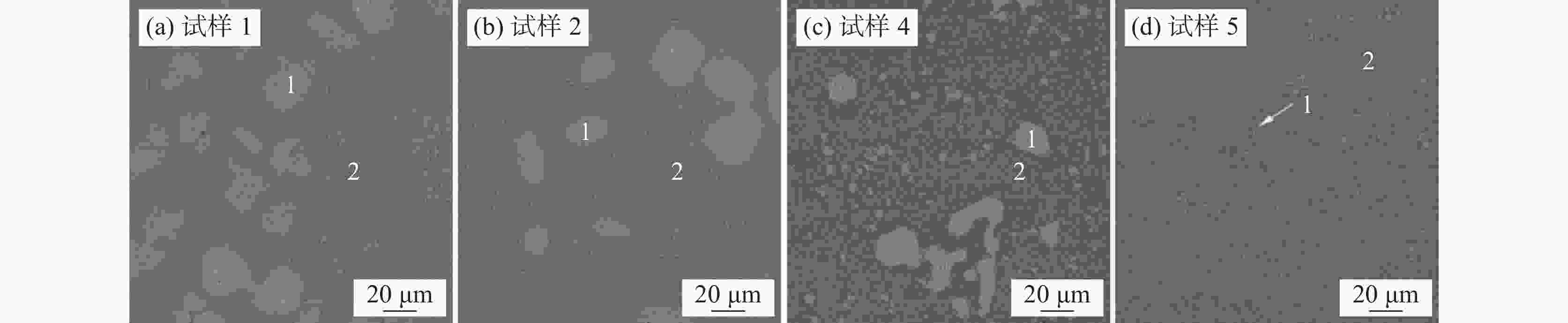
% FeO还原率 FeO SiO2 V2O3 Cr2O3 TiO2 MnO CaO 0 37 17 13 5 9 11 8 20 30 22 14 5 9 11 9 40 24 27 14 5 9 11 10 60 18 32 15 5 9 11 10 80 11 37 16 5 9 11 11 表 4 硅酸盐熔体中显微结构单元分类
Table 4. Classification of microstructural units in silicate melts
骨架型式 组成单元 NBO/T Qi 三维网络 SiO2 0 Q4 层状、板状 Si2O52− 1 Q3 链、环型 Si2O64− 2 Q2 二聚体 Si2O76− 3 Q1 孤立体 SiO44− 4 Q0 -
[1] He Simiao, Yuan Shouqiian, Zhu Lifang. Research status of recycling of crystal silicon cutting waste[J]. Chemical Industry and Engineering Progress, 2013,32(4):925−929. (何思邈, 袁守谦, 朱丽芳. 晶体硅切割废料回收的研究现状[J]. 化工进展, 2013,32(4):925−929. [2] Bruton T M. General trends about photovoltaic basedon crystalline silicon[J]. Solar Energy Materials and Solar Cells, 2002,72(1):3−10. [3] Liu Fangxu. Research on the current situation and development strategy of China's photovoltaic industry[J]. Technology and Economic Guide, 2019,27(30):16−17. (刘方旭. 中国光伏产业现状与发展策略研究[J]. 科技经济导刊, 2019,27(30):16−17. [4] Xing Pengfei, Guo Zhu, Liu Yan, et al. Recovery of cutting waste slurry of monocrystalline silicon and polycrystalline silicon[J]. Journal of Materials and Metallurgy, 2010,9(2):148−153. (邢鹏飞, 郭著, 刘燕, 等. 单晶硅和多晶硅切割废料浆的回收[J]. 材料与冶金学报, 2010,9(2):148−153. doi: 10.3969/j.issn.1671-6620.2010.02.016 [5] 曾晓兰. 钒渣物化性质与相图研究[D]. 重庆: 重庆大学, 2012.Zeng Xiaolan. Study on physicochemical properties and phase diagram of vanadium slag[D]. Chongqing: Chongqing University, 2012. [6] 赵月浩. 含铬型钒渣熔化特性及熔体结构研究[D]. 沈阳: 东北大学, 2015.Zhao Yuehao. Study on melting characteristics and melt structure of chromium containing vanadium slag[D]. Shenyang: Northeast University, 2015. [7] 毛裕文. 冶金熔体[M]. 北京: 冶金工业出版社, 1994: 210−223.Mao Yuwen. Metallurgical melt [M]. Beijing: Metallurgical Industry Press, 1994: 210−223. [8] Zhang Baichuan. Direct smelting of ferrovanadium with vanadium slag[J]. Ferroalloy, 1979,10(2):30−36. (张百川. 钒渣直接冶炼钒铁[J]. 铁合金, 1979,10(2):30−36. [9] 田键. 硅酸盐晶体学[M]. 武汉: 武汉大学出版社, 2010: 54−59.Tian Jian. Silicate crystallography [M]. Wuhan: Wuhan University Press, 2010: 54−59. [10] 张燮. FeO-SiO2-V2O3渣系熔融结构和性质的多尺度分析[D]. 重庆: 重庆大学, 2012.Zhang Xie. Multiscale analysis of melting structure and properties of FeO-SiO2-V2O3 slag system[D]. Chongqing: Chongqing University, 2012. -




 下载:
下载: