Study on leaching kinetics of titanium from waste denitrification catalyst with salt roasting-acid leaching
-
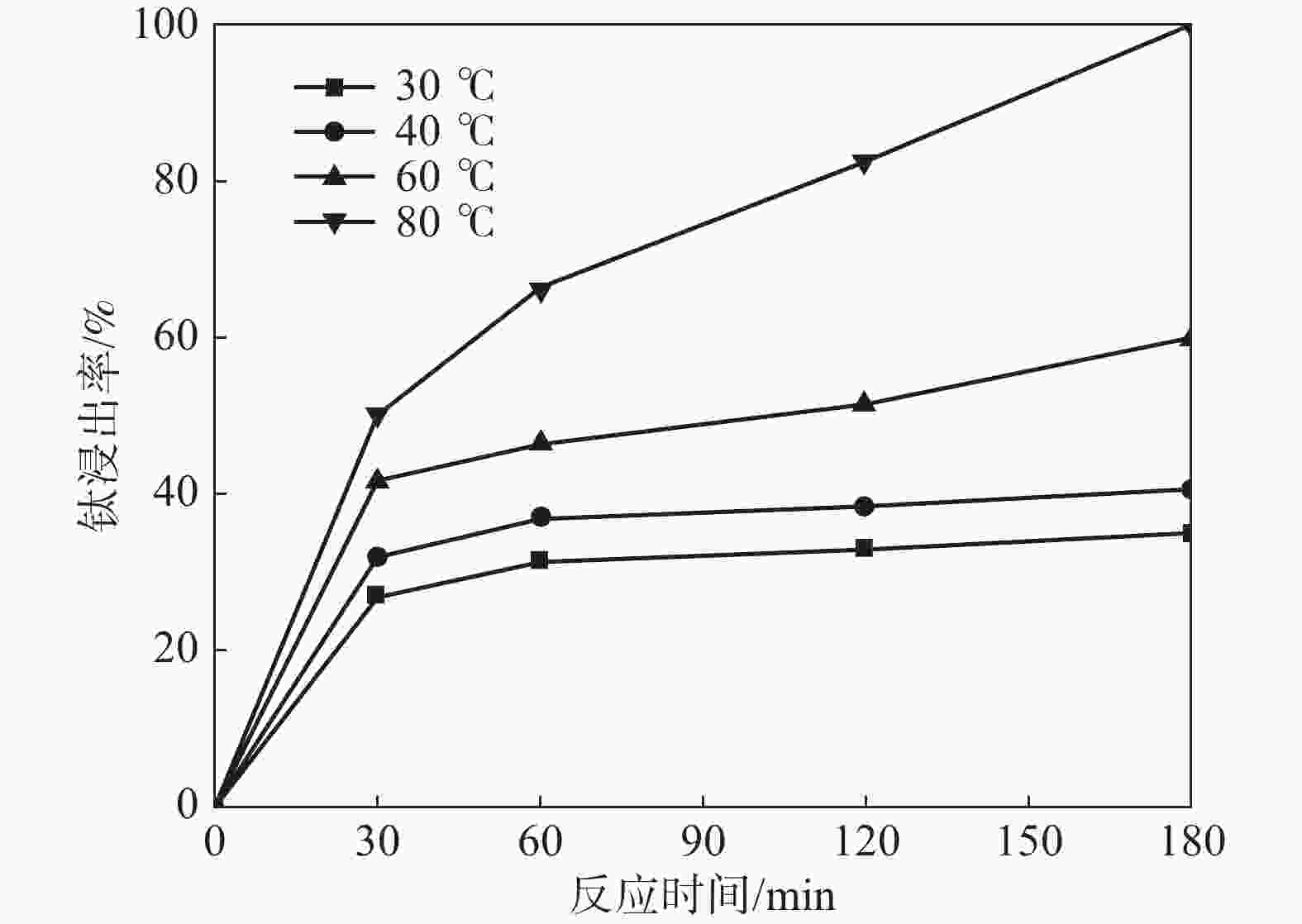
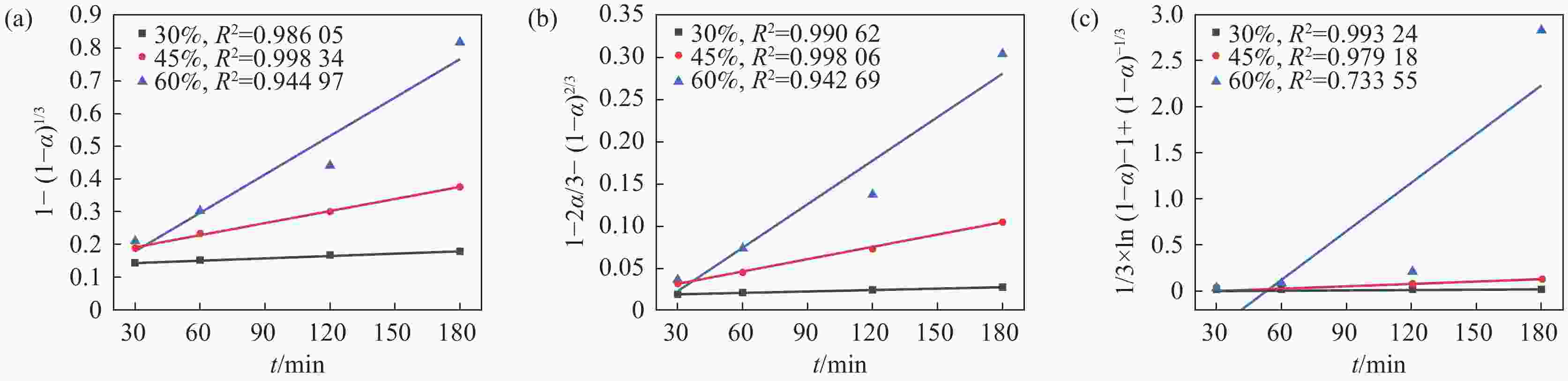
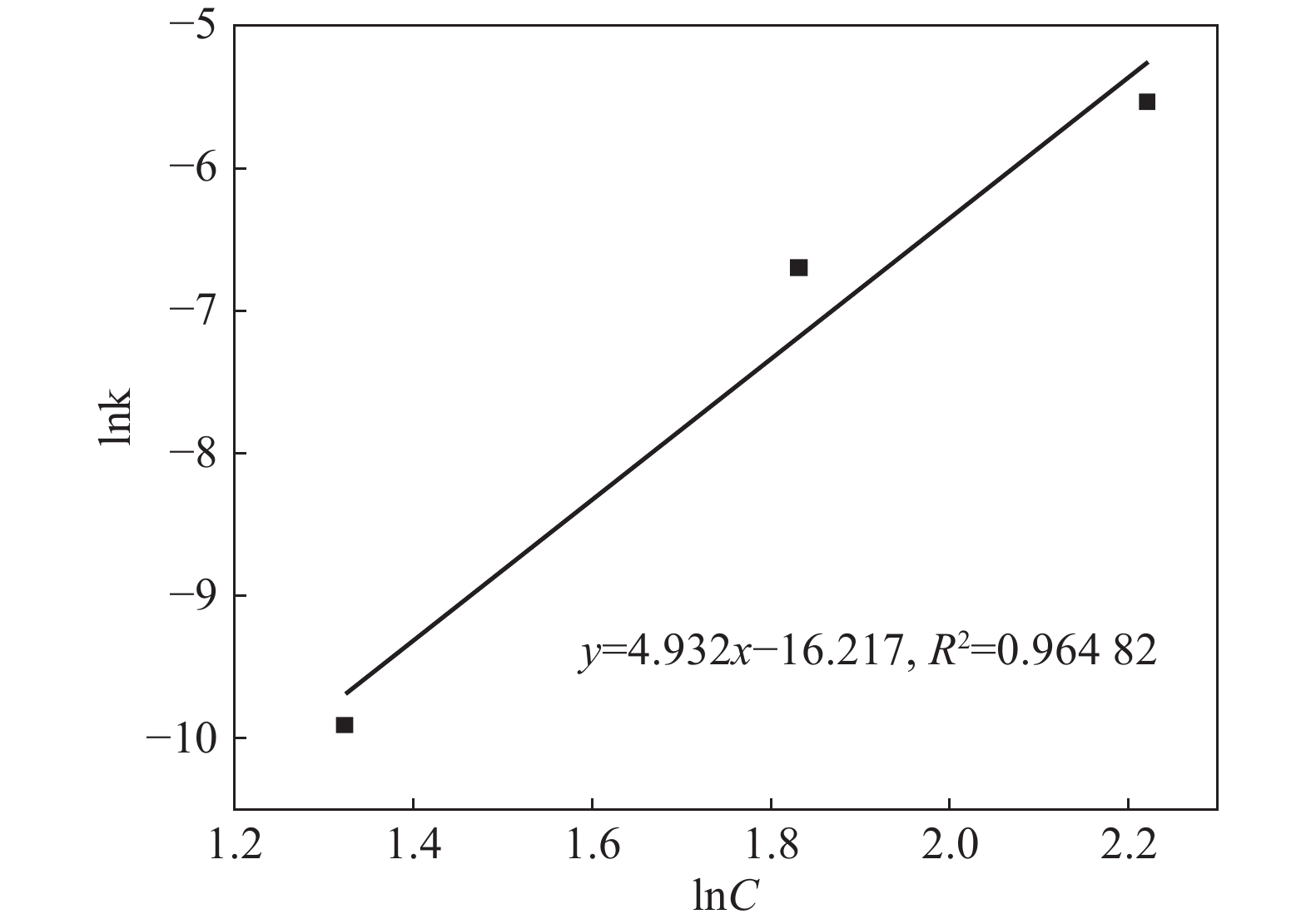
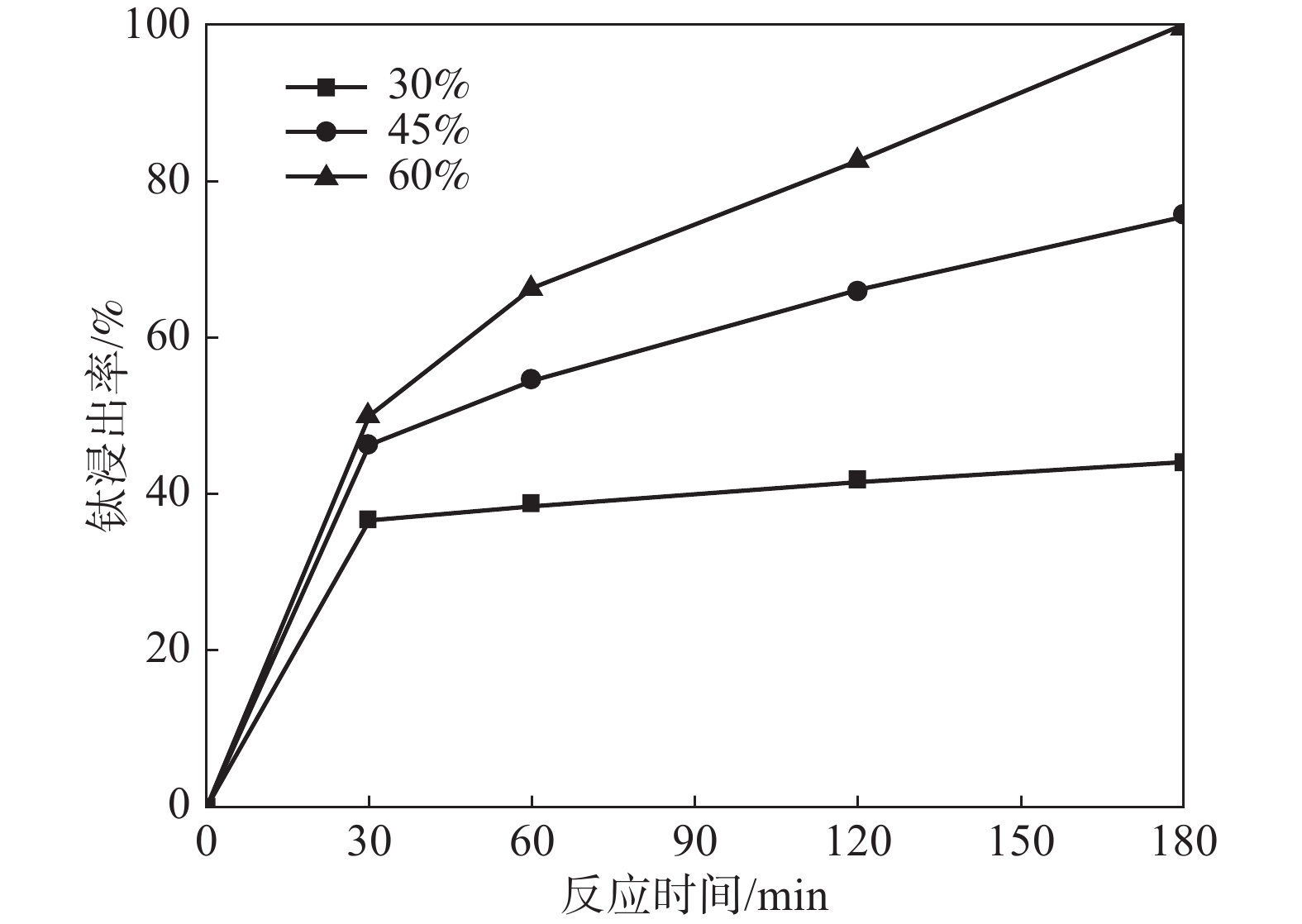
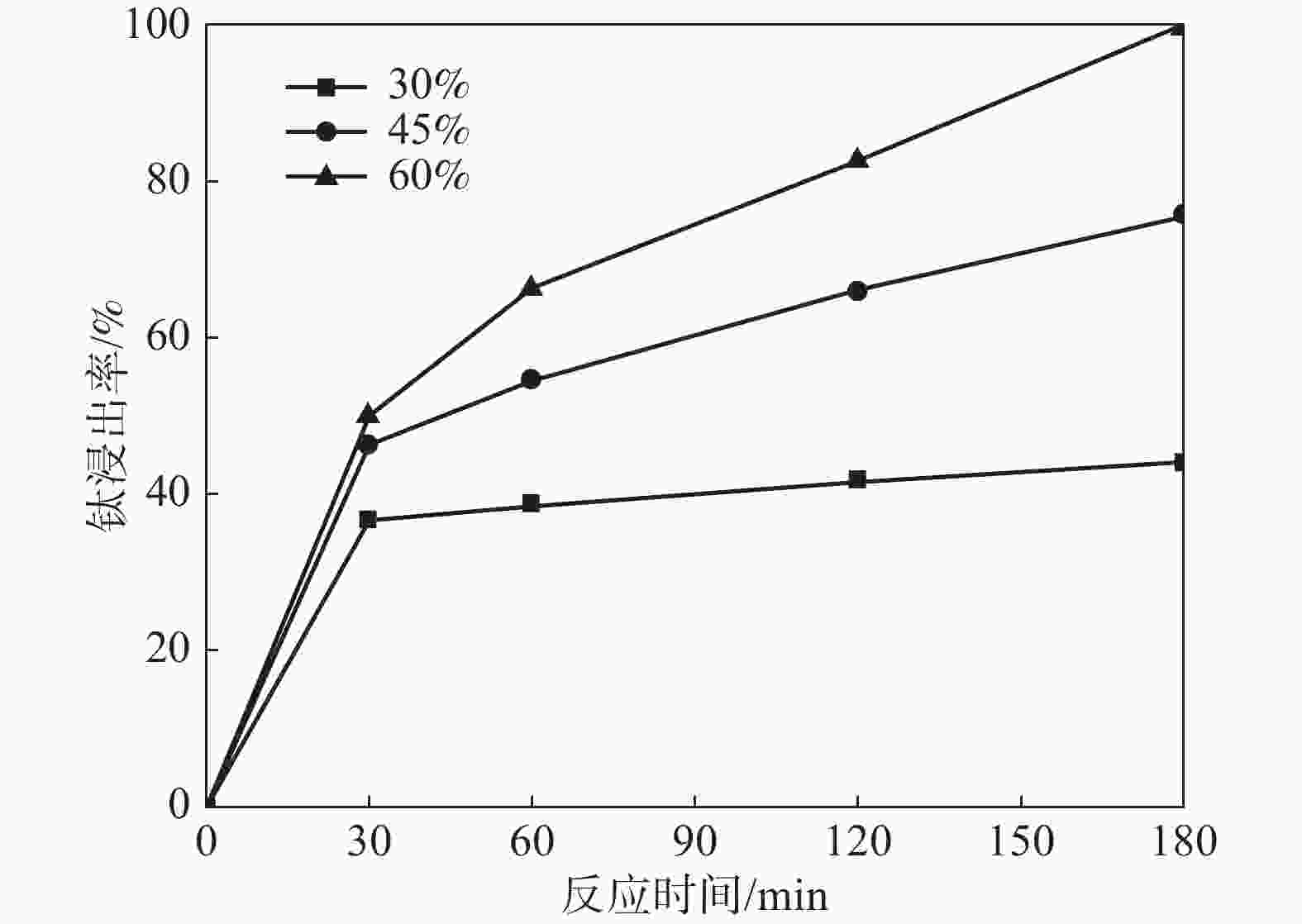
摘要: 采用加盐焙烧-酸浸法回收废脱硝催化剂中的钛,利用液-固多相反应的核收缩模型研究硫酸浸钛的浸出动力学,考察了硫酸浓度和酸浸温度对钛浸出反应速率的影响。结果表明,在温度低于60 ℃或硫酸质量分数小于45%时,浸出受化学反应和固膜扩散混合控制;升温和提高硫酸浓度浸出过程则转变为化学反应控制。低温受混合控制时的表观活化能为30.23 kJ/mol,升温后受化学反应控制时的表观活化能为92.92 kJ/mol,表观反应级数为4.932。提高反应温度和硫酸浓度均能加快钛的浸出速率,提高钛的浸出率。Abstract: Titanium from waste denitrification catalyst was recovered by salt roasting-acid leaching method. The leaching kinetics of titanium in sulfuric acid was studied by nuclear shrinkage model of liquid-solid multiphase reaction. The effects of sulfuric acid concentration and acid leaching temperature on the leaching rate of titanium were investigated. The results show that when the temperature is lower than 60 ℃ or the mass fraction of sulfuric acid is less than 45%, the leaching process is controlled by chemical reaction and solid film diffusion. The leaching process of heating up and increasing sulfuric acid concentration is controlled only by chemical reaction. The apparent activation energy is 30.23 kJ/mol under mixed control at low temperature, and 92.92 kJ/mol under chemical control at high temperature, and the apparent reaction order is 4.932. Increasing the reaction temperature and sulfuric acid concentration can accelerate the leaching rate of titanium and improve the leaching rate of titanium.
-
Key words:
- spent denitrification catalyst /
- titanium recovery /
- salt roasting-acid leaching /
- leaching /
- kinetics
-
表 1 废催化剂主要氧化物成分
Table 1. Main oxide composition of spent catalyst
% V2O5 TiO2 Al2O3 SiO2 Na2O CaO 1.149 2.752 45.382 45.741 0.141 0.165 -
[1] Wang Xiuwen, Li Lulu, Sun Jingfang, et al. Analysis of NOx emission and control in China and research progress in denitration catalysts.[J]. Industrial Catalysis, 2019,27(2):1−23. (王修文, 李露露, 孙敬方, 等. 我国氮氧化物排放控制及脱硝催化剂研究进展[J]. 工业催化, 2019,27(2):1−23. doi: 10.3969/j.issn.1008-1143.2019.02.001Wang Xiuwen, Li Lulu, Sun Jingfang, et al. Research progress of nox emissioncontrol and denitrification catalysts inChina[J]. Industrial Catalysis, 2019, 27(2): 1-23 doi: 10.3969/j.issn.1008-1143.2019.02.001 [2] Wang Qiubao, Liu Zhiyuan. Influence of thermal power plant on atmosphere and its prevention[J]. Electronic Test, 2016,(23):163−165. (王秋堡, 刘知远. 火力发电厂对大气的影响与防治[J]. 电子测试, 2016,(23):163−165. doi: 10.3969/j.issn.1000-8519.2016.23.097Wang Qiubao, Liu Zhiyuan. Influence of thermal power plant on atmosphere and its prevention[J]. Electronic Test, 2016(23): 163-165. doi: 10.3969/j.issn.1000-8519.2016.23.097 [3] Wang Chunlan, Song Hao, Han Dongqin. Development and application of SCR catalyst regeneration technology for denitrification[J]. China's Environmental Protection Industry, 2014,(4):22−25. (王春兰, 宋浩, 韩东琴. SCR脱硝催化剂再生技术的发展及应用[J]. 中国环保产业, 2014,(4):22−25. doi: 10.3969/j.issn.1006-5377.2014.04.006Wang Chunlan, Song Hao, Han Dongqin. Development and application of SCR catalyst regeneration technology for denitrification[J]. China's Environmental Protection Industry, 2014(4): 22-25. doi: 10.3969/j.issn.1006-5377.2014.04.006 [4] Huang Jiehui, Wu Junfeng, Ren Xiaoming, et al. Recycling and environmental management of spent SCR denitrification catalyst[J]. Environmental Science and Technology, 2015,28(6):74−77. (黄洁慧, 吴俊锋, 任晓鸣, 等. 废SCR脱硝催化剂的再生回收及环境管理[J]. 环境科技, 2015,28(6):74−77. doi: 10.3969/j.issn.1674-4829.2015.06.017Huang Jiehui, Wu Junfeng, Ren Xiaoming, et al. Recycling and environmental management of spent SCR denitrification catalyst[J]. Environmental Science and Technology, 2015, 28(6): 74-77. doi: 10.3969/j.issn.1674-4829.2015.06.017 [5] Guo Lixin, Lu Yihai, Han Zhongge. Discussion on deactivation mechanism and regeneration technology of SCR denitrification catalyst in thermal power plant[J]. Resource Conservation and Environmental Protection, 2016,(8):19−20. (郭力欣, 陆义海, 韩忠阁. 火电厂SCR脱硝催化剂失活机理与再生技术探讨[J]. 资源节约与环保, 2016,(8):19−20. doi: 10.3969/j.issn.1673-2251.2016.08.022Guo Lixin, Lu Yihai, Han Zhongge. Discussion on deactivation mechanism and regeneration technology of SCR denitrification catalyst in thermal power plant[J]. Resource Conservation and Environmental Protection, 2016(8): 19-20. doi: 10.3969/j.issn.1673-2251.2016.08.022 [6] Cao Limei, Wang Qing, Zhang Wei, et al. Analysis and environmental management of SCR catalyst for typical coal-fired power plant waste[J]. Equipment Environmental Engineering, 2018,15(2):45−51. (曹礼梅, 王青, 张巍, 等. 典型燃煤电厂废SCR催化剂解析及环境管理思考[J]. 装备环境工程, 2018,15(2):45−51.Cao Limei, Wang Qing, Zhang Wei, et al. Analysis and environmental management of SCR catalyst for typical coal-fired power plant waste[J]. Equipment EnvironmentalEngineering, 2018, 15(2): 45-51. [7] Zhang Tao, Chen Xiaoli, Sun Chao, et al. Research progress on valuable metal recovery and reuse of waste vanadium titanium SCR catalyst[J]. Modern Chemical Industry, 2021,41(S1):67−72,77. (张涛, 陈晓利, 孙超, 等. 废钒钛系SCR催化剂有价金属回收与再利用研究进展[J]. 现代化工, 2021,41(S1):67−72,77.Zhang Tao, Chen Xiaoli, Sun Chao, et al. Research progress on valuable metal recovery and reuse of waste vanadium titanium SCR catalyst[J]. Modern Chemical Industry, 2021, 41(S1): 67-72, 77. [8] Gao Zhaowei, Cao Chengchao, Li Yaoshan, et al. Research on acid leaching kinetics of high calcium low grade copper ore[J]. Mining and Metallurgical Engineering, 2021,41(6):170−173. (高昭伟, 曹成超, 李耀山, 等. 高钙型低品位铜矿酸性浸出动力学研究[J]. 矿冶工程, 2021,41(6):170−173. doi: 10.3969/j.issn.0253-6099.2021.06.041Gao Zhaowei, Cao Chengchao, Li Yaoshan, et al. Research on acid leaching kinetics of high calcium low grade copper ore[J]. Mining and Metallurgical Engineering, 2021, 41(6): 170-173. doi: 10.3969/j.issn.0253-6099.2021.06.041 [9] Yang Lijiao, Chen Nanchun, Zhong Xiaping, et al. Kinetic analysis of lead leaching from lead slag in nacl-hcl system[J]. The Chinese Journal of Nonferrous Metals, 2015,25(6):1705−1712. (杨利姣, 陈南春, 钟夏平, 等. NaCl-HCl体系浸出铅渣中铅的动力学分析[J]. 中国有色金属学报, 2015,25(6):1705−1712.Yang Lijiao, Chen Nanchun, Zhong Xiaping, et al. Kinetic analysis of lead leaching from lead slag in nacl-hcl system[J]. The Chinese Journal of Nonferrous Metals, 2015, 25(6): 1705-1712. [10] Jiang Yan, Sun Lida, Li Zijing, et al. Study on leaching kinetics of zinc from blast furnace dust[J]. Inorganic Chemicals Industry, 2015,47(1):53−55. (姜艳, 孙丽达, 李自静, 等. 高炉烟尘中锌的浸出动力学研究[J]. 无机盐工业, 2015,47(1):53−55.Jiang Yan, Sun Lida, Li Zijing, et al. Study on leaching kinetics of zinc from blast furnace dust[J]. Inorganic Chemicals Industry, 2015, 47(1): 53-55. -




 下载:
下载: