Preparation and characterization of titanium dioxide as catalyst support for denitration
-
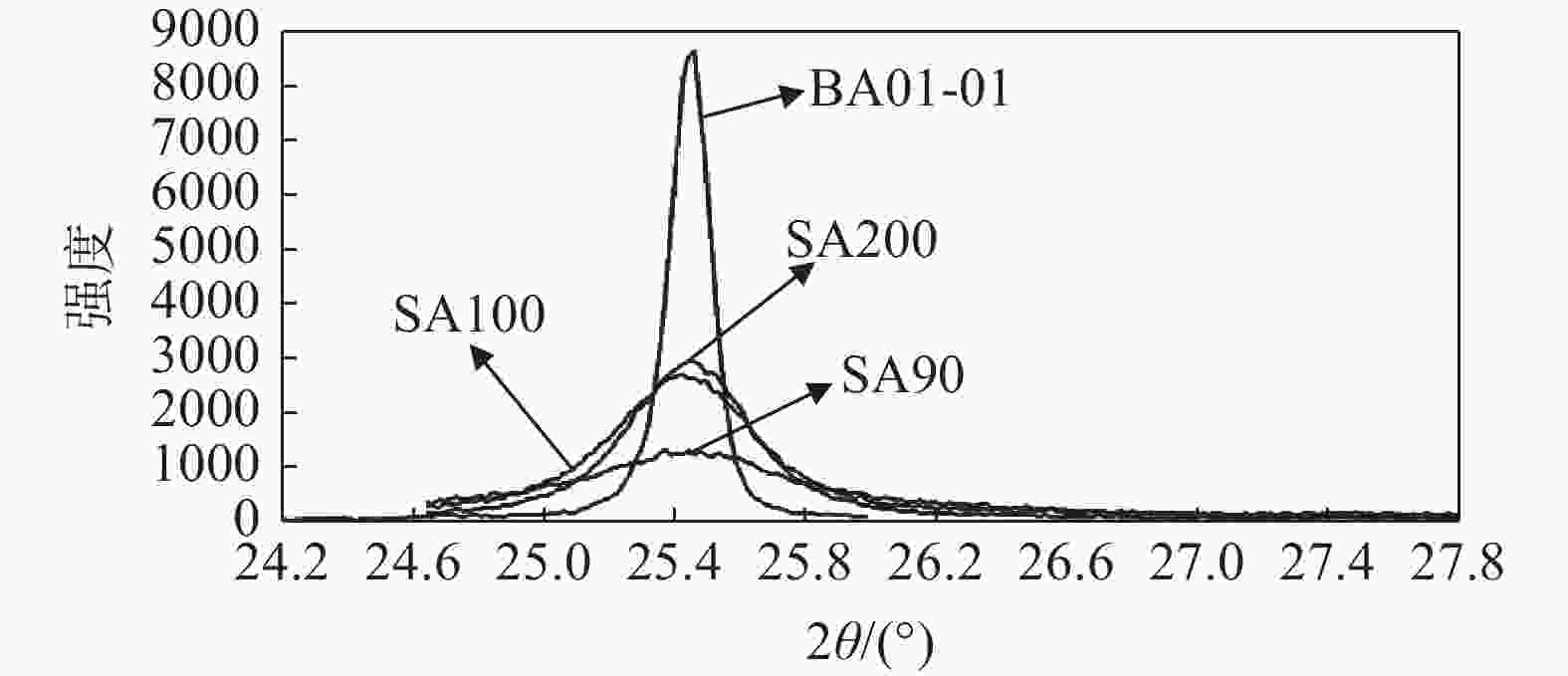
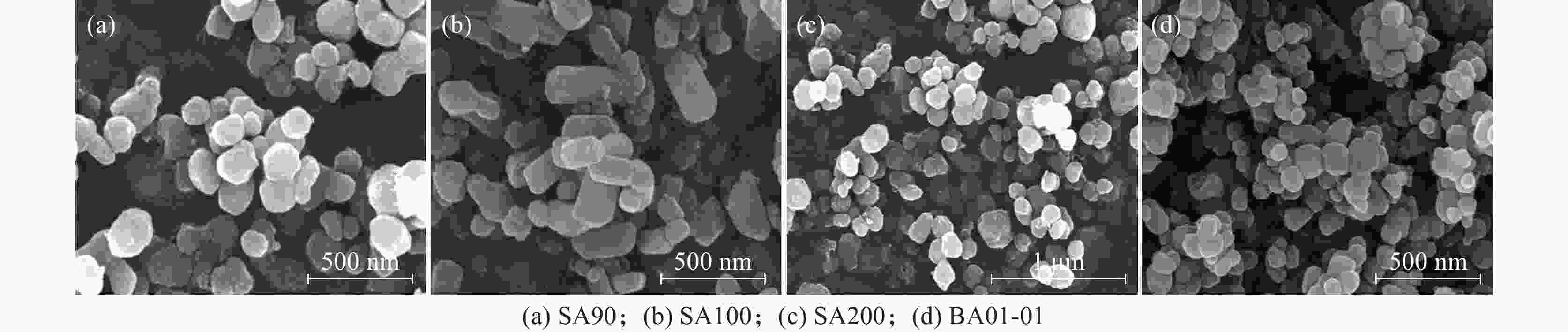
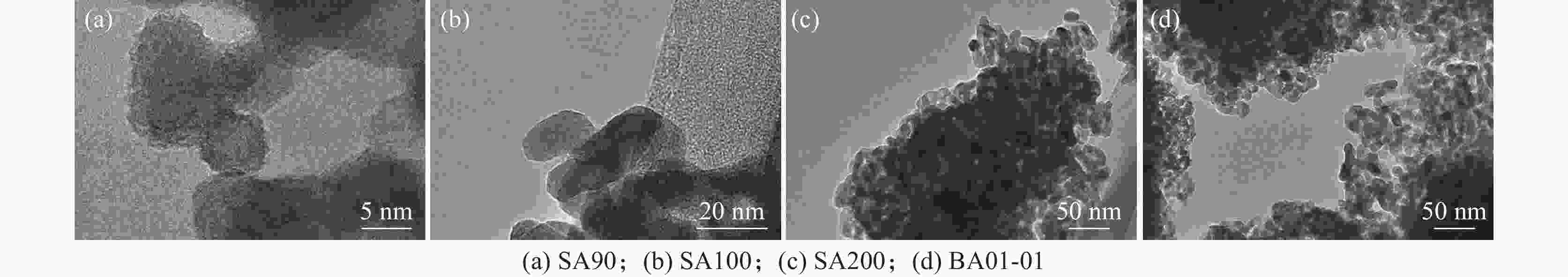
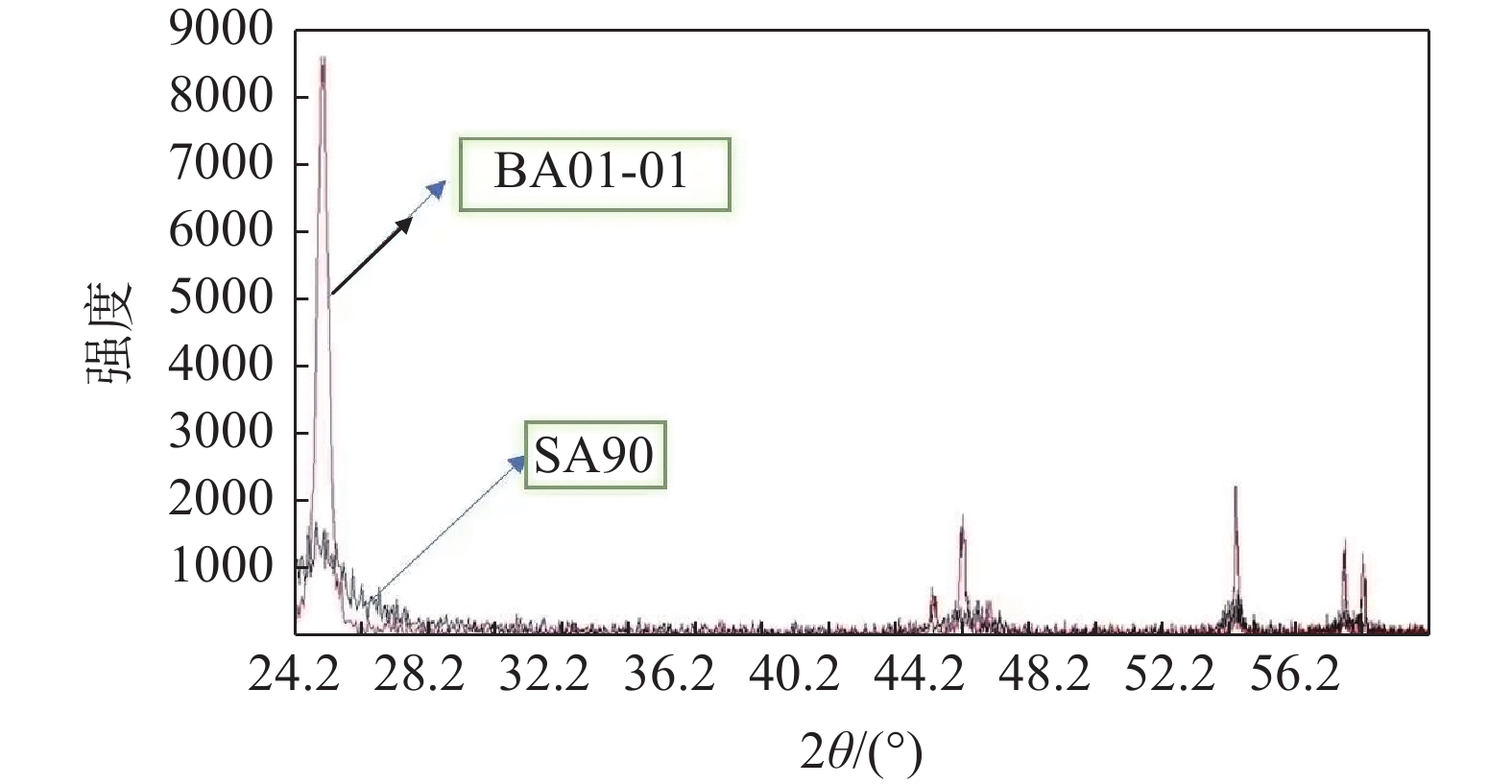
摘要: 以硫酸法钛白粉生产的偏钛酸为原料,经过氨水中和,活性元素复合、煅烧、粉碎等工艺过程,制备了脱硝催化剂载体二氧化钛。利用粒度分布、XRD、SEM等测试手段对粉体的晶型结构、形貌等进行表征。结果表明制备的脱硝催化剂载体二氧化钛为锐钛型,粒度分布均匀,颗粒形貌较好;随着煅烧温度的升高,制备的脱硝催化剂载体二氧化钛SA90、SA100、SA200的衍射峰的强度都远低于纯锐钛型钛白粉样品BA01-01的衍射峰,结晶尺寸在100~200 nm,活性较好。Abstract: The specific titanium dioxide, a catalyst carrier for denitration, was prepared from metatitanic acid in the process of sulfuric acid titanium dioxide production, via ammonia neutralization, active element complexing, calcination and pulverization. The crystal structure and morphology of the powders were characterized by means of particle size distribution, XRD and SEM. The results show that the as-prepared titanium dioxide for denitration catalyst support is anatase type with uniform particle size distribution and good particle morphology. With the increase of calcination temperature, the diffraction peaks of the as-prepared denitration catalyst support TiO2 SA90, SA100 and SA200 are much lower than those of pure anatase titanium dioxide sample BA01-01. The crystal size is in the range of 100-200 nm, and the activity is good.
-
Key words:
- denitration catalyst /
- titanium dioxide /
- metantitanic acid /
- crystal type /
- crystal size
-
表 1 制备脱硝催化剂载体二氧化钛与标准锐钛型钛白粉和金红石型钛白粉的晶粒尺寸
Table 1. Grain size of denitration catalyst support TiO2 and standard anatase TiO2 and rutile
nm SA90 SA100 SA200 BA01-01 金红石型 107 171 150 494 521 表 2 脱硝催化剂载体二氧化钛与锐钛型钛白粉的BET与粒度分布
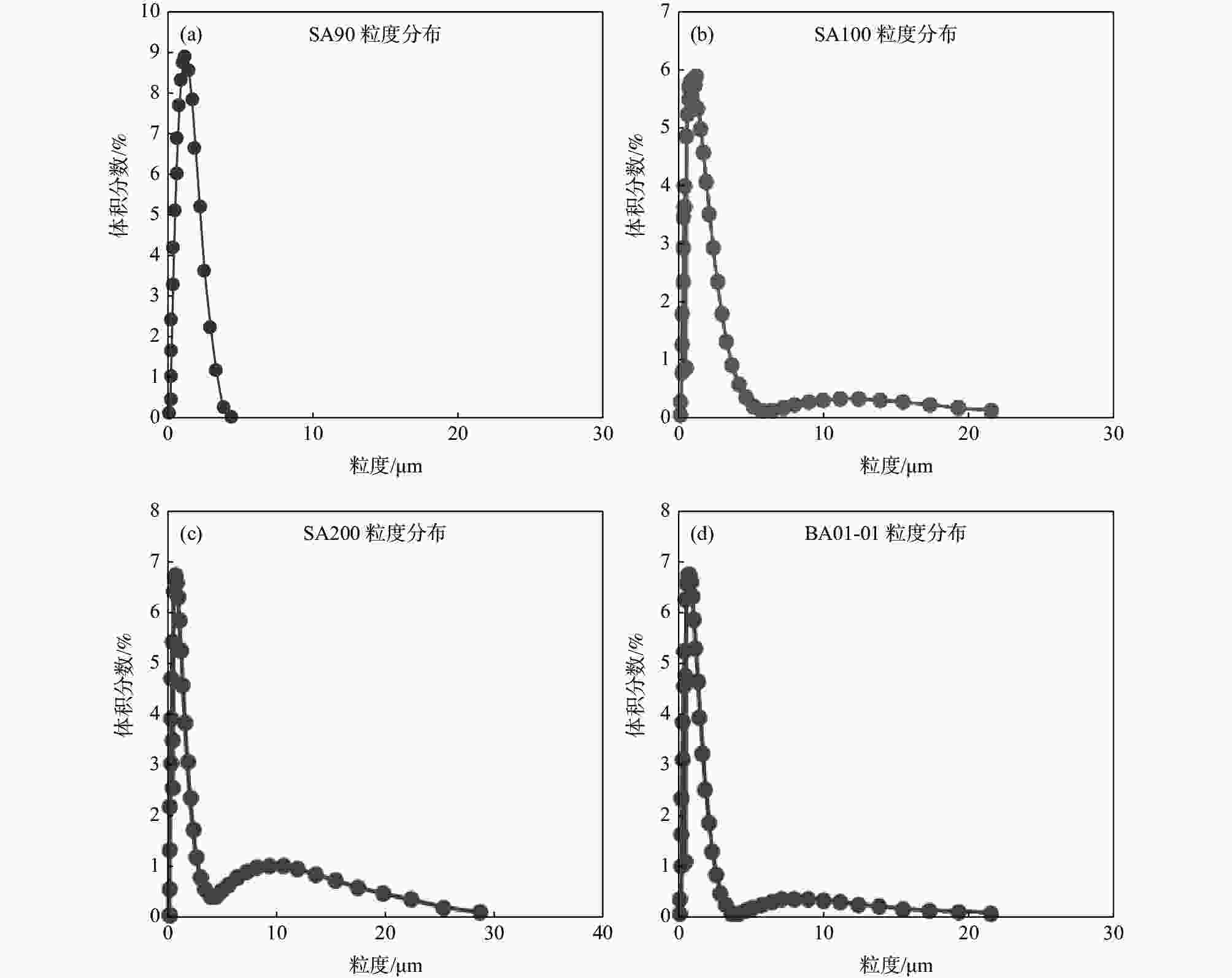
Table 2. BET and particle size distribution of denitration catalyst support TiO2 and anatase TiO2
样品 BET/(m2·g−1) D10/μm D50/μm D90/μm SA90 107.00 0.461 1.049 2.087 SA100 107.92 0.383 0.93 2.360 SA200 108.46 0.359 0.822 4.218 BA01-01 9.25 0.351 0.750 1.719 表 3 本研究制备的脱硝催化剂载体二氧化钛与国外产品的理化性能比较
Table 3. Physical and chemical properties comparison between the as-prepared denitration catalyst support TiO2 and some foreign products
样品 水分/% 游离(SO42−)/% 总(SO42−)/% TiO2/% R/% 晶粒度/nm SA90 4.1 3.51 5.13 91.3 0.11 12.1 MC-9 4.0 3.55 5.20 91.0 0.20 13.0 SA100 1.17 3.03 4.45 92.6 0.22 11.2 MC-9-L 1.35 3.00 4.50 92.0 0.30 12.0 SA200 0.4 2.79 3.52 95.3 0.26 9.2 MC-5 0.5 2.80 3.50 95.5 0.30 11.0 -
[1] Zhu Xiaodong, Song Huijin, Wang Mingkun, et al. Preparation and characterization of anatase Nd-doped TiO2 with high thermal stability[J]. Jouranal of Synthetic Crystals, 2016,45(8):2147−2151. (朱晓东, 宋慧瑾, 王明坤, 等. 高热稳定性锐钛矿型Nb掺杂纳米TiO2的制备与表征[J]. 人工晶体学报, 2016,45(8):2147−2151. doi: 10.3969/j.issn.1000-985X.2016.08.029Zhu Xiaodong, Song Huijin, Wang Mingkun, et al. Preparation and characterization of anatase Nd-doped TiO2 with high thermal stability[J]. Jouranal of Synthetic Crystals, 2016, 45(8): 2147-2151. doi: 10.3969/j.issn.1000-985X.2016.08.029 [2] Buxbaum G, Pfaff G. Industrial inorganic pigments[M]. WILEY-VCH Verlag GmbH& Co. KGaA, Weinheim, 2005. [3] Jalava J P. The use of an exact light-scattering theory for spheroidal TiO2 pigment partricles[J]. Part & Particle Systems Characterization, 2006,23(2):159−164. [4] Wang Y, Li J, Wang L, et al. Preparation of rutiletitanium dioxide white pigment via doping and calcination of metatitanate acid obtained by the NaOH molten salt method[J]. Industrial & Engineering Chemistry Research, 2010,49(16):7693−7696. [5] Ansari Sajid Ali, Khan Mohammad Mansoob, Ansari Mohd Omaish, et al. Silver nanoparticles and defect-induced visible light photocatalytic and photoelectrochemical performance of Ag@m-TiO2 nanocomposite[J]. Solar Energy Materials and Solar Cells, 2015,141:162−170. doi: 10.1016/j.solmat.2015.05.029 [6] Ganesan Srividhya, Muruganandham Abinaya, Mounasamy Veena, et al. Highly selective dimethylamine sensing performance of TiO2 thin films at room temperature[J]. Journal of Nanoscience and Naotechnology, 2020,20(5):3131−3139. doi: 10.1166/jnn.2020.17199 [7] Cao Lingyun, Fei Xuening, Zhao Hongbin, et al. Preparation of phthalocyanine blue/rutile TiO2 composite pigment with a ball milling method and study on its NIR reflectivity[J]. Dye and Pigments, 2020,173:107898. doi: 10.1016/j.dyepig.2019.107898 [8] Li Dandan, Yao Guangzhen, Liang Guiyan, et al. Preparation of Go/TiO2 composite photocatalyst and treatment of synthetic dye wastewater[J]. Journal of Materials Engineering, 2019,47(12):104−110. (李丹丹, 姚广铮, 梁桂琰, 等. 氧化石墨烯复合二氧化钛光催化剂的制备及模拟染料废水处理[J]. 材料工程, 2019,47(12):104−110. doi: 10.11868/j.issn.1001-4381.2018.000701Li Dandan, Yao Guangzhen, Liang Guiyan, et al. Preparation of Go/TiO2 composite photocatalyst and treatment of synthetic dye wastewater[J]. Journal of Materials Engineering, 2019, 47(12): 104-110. doi: 10.11868/j.issn.1001-4381.2018.000701 [9] Chong Mengnan, Jin Bo, Chow Christopher W K. Recent developments in photocatalytic water treatment technology: A review[J]. Water Research, 2010,44(10):2997−3027. doi: 10.1016/j.watres.2010.02.039 [10] Wang Lina, Li Zhigang. The present situation and mechanisms of NOx removal technology from flue gas[J]. Shandong Electric Power, 2010,174(3):62−65. (王丽娜, 李治钢. 烟气脱氮技术机理及研究现状[J]. 山东电力技术, 2010,174(3):62−65.Wang Lina, Li Zhigang. The present situation and mechanisms of NOx removal technology from flue gas[J]. Shandong Electric Power, 2010, 174(3): 62-65. [11] Hu Y H, Griffiths K, Norton P R. Surface science studies of selective catalytic reduction of NO: Progress in the last ten years[J]. Surface Science, 2009,603(10-12):1740−1750. doi: 10.1016/j.susc.2008.09.051 [12] Lisi L, Lasorella G, Malloggi S, et al. Single and combined deactivating effect of alkali metals and HCl on commercial SCR catalysts[J]. Applied Catalysis B:Environmental, 2004,50(4):251−258. doi: 10.1016/j.apcatb.2004.01.007 [13] Gu Min, Yang Jia, Du Yungui. Effect of titanium dioxide support on the properties for selective catalytic reduction de-NOx catalyst[J]. Guangzhou Chemistry, 2012,37(4):49−54. (辜敏, 杨佳, 杜云贵. 钛白粉载体对选择催化还原脱硝催化剂性能的影响[J]. 广州化学, 2012,37(4):49−54. doi: 10.3969/j.issn.1009-220X.2012.04.009Gu Min, Yang Jia, Du Yungui. Effect of titanium dioxide support on the properties for selective catalytic reduction de-NOx catalyst[J]. Guangzhou Chemistry, 2012, 37(4): 49-54. doi: 10.3969/j.issn.1009-220X.2012.04.009 [14] Zhou Tao, Liu Shaoguang, Tang Mingzao, et al. Research progress on selective catalytic reduction de-NOx catalysts[J]. Journal of the Chinese Ceramic Society, 2009,37(2):317−324. (周涛, 刘少光, 唐名早, 等. 选择性催化还原脱硝催化剂研究进展[J]. 硅酸盐学报, 2009,37(2):317−324. doi: 10.3321/j.issn:0454-5648.2009.02.029Zhou Tao, Liu Shaoguang, Tang Mingzao, et al. Research progress on selective catalytic reduction de-NOx catalysts[J]. Journal of the Chinese Ceramic Society, 2009, 37(2): 317-324. doi: 10.3321/j.issn:0454-5648.2009.02.029 [15] 孙从丽. 粒径均匀的脱硝催化剂载体纳米二氧化钛的制备[D]. 沈阳: 东北师范大学, 2015.Sun Congli. The preparation of nanometer titanium dioxide used for denitration catalyst supportor with uniform particle size[D]. Shengyang: Northeast Normal University, 2015. [16] Chang Hong, Wang Jinggang. Application of nanocrystalline titania in environmental protection field[J]. Mining & Metallurgy, 2002,11(4):73−76. (常虹, 王京刚. 纳米二氧化钛在环保领域中的应用[J]. 矿冶, 2002,11(4):73−76. doi: 10.3969/j.issn.1005-7854.2002.04.019Chang Hong, Wang Jinggang. Application of nanocrystalline titania in environmental protection field[J]. Mining & Metallurgy, 2002, 11(4): 73-76. doi: 10.3969/j.issn.1005-7854.2002.04.019 [17] Chao S, Petrovsky V, Dogan F. Effects of calcination on the microstructure and dielectric properpties of titanium dioxide ceramics[J]. J. Mater. Sci., 2010,(45):6685−6693. [18] Alam M J, Cameron D C. Preparation and characterization of TiO2 thin films by sol-gel method[J]. Journal of Sol-gel Science and Technology, 2002,2592:137−145. [19] Ren Jian, Li Guangzhao, Han Rui, et al. In-situ preparation of reduced graphene oxide/titanium dioxide composites by sol-gel method and their photocatalytic properties[J]. Journal of Functional Materials, 2019,7:7185−7190. [20] Yang L X, Luo S L, Liu S H, et al. Graphitized carbon nanotubes formed in TiO2 nanotube arrays: A novel functional material with tube-in-tube nanostructure[J]. The Journal of Physical Chemistry C, 2008,112:8939−8943. doi: 10.1021/jp8020613 [21] Kim D S, Kwak S Y. The hydrothermal synthesis of mesoporous TiO2 with high crystallinity, thermal stability, large surface area, and enhanced photocatalytic activity[J]. Appl. Gatal. Gen., 2007,323:110−118. doi: 10.1016/j.apcata.2007.02.010 [22] You Jia, Jiang Huan, Han Yanlin, et al. Study on preparation of CdS/TiO2 compositeby microemulsion and its photocatalytic properties[J]. Iron Steel Vanadium Titanium, 2020,41(1):24−31. (尤佳, 江环, 韩炎霖, 等. 微乳液法制备CdS/TiO2复合材料及光催化性能研究[J]. 钢铁钒钛, 2020,41(1):24−31. doi: 10.7513/j.issn.1004-7638.2020.01.005You Jia, Jiang Huan, Han Yanlin, et al. Study on preparation of CdS/TiO2 compositeby microemulsion and its photocatalytic properties[J]. Iron Steel Vanadium Titanium, 2020, 41(1): 24-31. doi: 10.7513/j.issn.1004-7638.2020.01.005 [23] Andronic L, Duta A. TiO2 thin films for dyes photodegradation[J]. Thin Solid Films, 2007,515:6294−6297. doi: 10.1016/j.tsf.2006.11.150 -




 下载:
下载: