Thermodynamic study on effect of TiO2 addition on Al and Ti distribution during electroslag remelting of Incoloy825
-
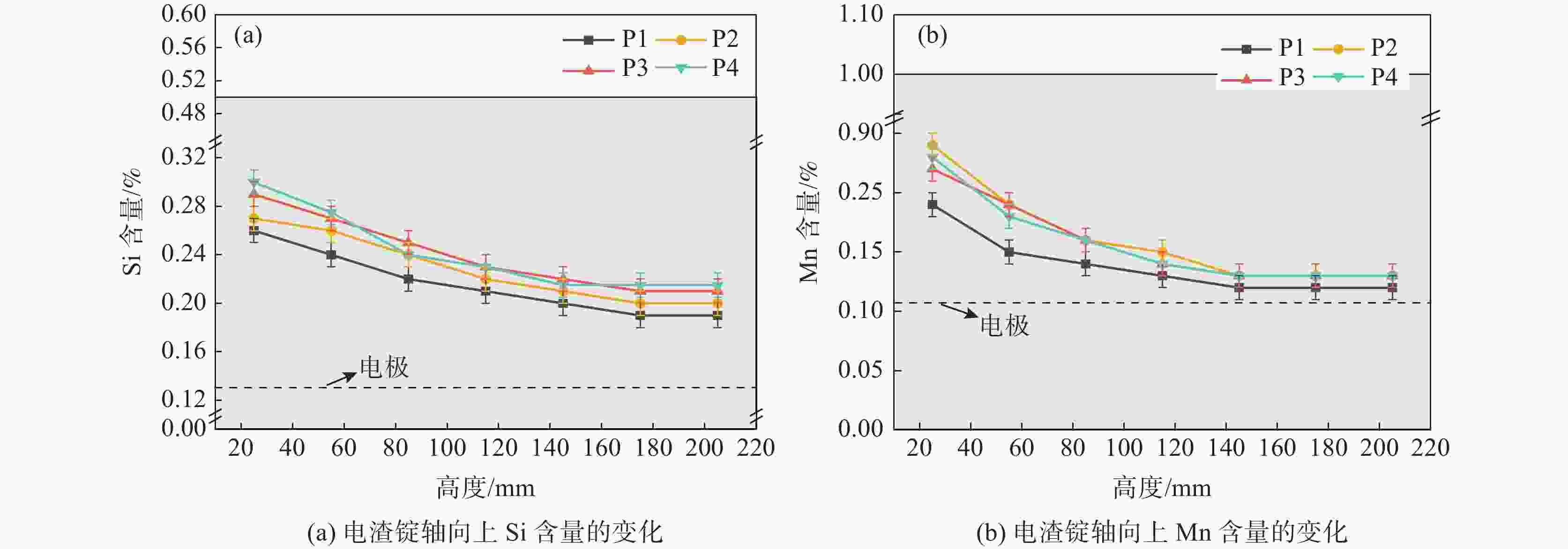
摘要: 为了研究低氟渣电渣重熔过程中电渣锭中元素的变化,以Incoloy825合金为研究对象,渣中添加不同含量的TiO2和脱氧剂,进行了四组电渣重熔试验;并基于离子分子共存理论、热力学理论和质量守恒定律建立Al、Ti含量控制的热力学模型。结果表明,随着渣中TiO2含量的增加,电渣锭中Ti含量增加,Al含量减少,这是由于铝钛的交换反应4Al+3TiO2=3Ti+2Al2O3控制的,Si和Mn元素含量变化不大。当TiO2含量不变时,Al、Ti元素的含量沿着电渣锭高度的方向上有不同程度的增加,Si、Mn元素的含量则均有所下降。当熔渣中
$ \mathrm{l}\mathrm{g}({a}_{{\mathrm{A}\mathrm{l}}_{2}{\mathrm{O}}_{3}}^{2}/{a}_{\mathrm{T}\mathrm{i}{\mathrm{O}}_{2}}^{2}) $ 为−3.16时,结合Al脱氧剂的添加,可以得到Al、Ti含量均匀性较好的产品,试验结果很好地验证了热力学模型的准确性。-
关键词:
- Incoloy825合金 /
- 电渣重熔 /
- TiO2 /
- 热力学模型
Abstract: To investigate the variation of elements in ingots produced by electroslag remelting in low-fluorine slag system, based on Incoloy825 different contents of TiO2 and deoxidizer were added into slag to carry out four sets of electroslag remelting experiments. A thermodynamic model was established based on the Ions and Molecule coexistence Theory (IMCT), thermodynamics theory and the mass conservation law to control Al and Ti contents. It is found out that increasing TiO2 addition into the slag brings increasing Ti content and declined Al content in ingots, which is attributed to exchange reaction of 4Al+3TiO2=3Ti+2Al2O3, but slight changes of Si and Mn contents. When the TiO2 content remains constant, the Al and Ti contents increase along the height of ingots, while Si and Mn contents decrease. When the$ \mathrm{l}\mathrm{g}({a}_{{\mathrm{A}\mathrm{l}}_{2}{\mathrm{O}}_{3}}^{2}/{a}_{\mathrm{T}\mathrm{i}{\mathrm{O}}_{2}}^{2}) $ in slag is at −3.16 in combination with addition of Al deoxidizer, the obtained product can achieve uniform Al and Ti distribution. The predicted model shows good agreement with experimental results.-
Key words:
- Incoloy825 /
- electroslag remelting /
- TiO2 /
- thermodynamic model
-
表 1 自耗电极主要化学成分
Table 1. Main chemical composition of consumable electrode
% C Mn Si P S Cr Mo Ni Cu Al Ti Fe 0.101 0.107 0.131 0.009 0.009 20.620 3.180 38.880 1.660 0.120 1.000 33.740 表 2 预熔渣成分
Table 2. Chemical component of premelting slag
% 编号 CaF2 CaO Al2O3 MgO TiO2 FeO SiO2 MnO S1 18.60 35.59 36.81 1.51 3.47 1.15 2.44 0.43 S2 18.15 33.51 35.85 1.61 7.04 1.16 2.26 0.42 S3 18.09 30.75 33.53 1.47 11.84 1.27 2.65 0.40 注:S4渣是在S3渣系基础上加入50 g的Al脱氧剂。 表 3 电渣锭轴向上渣含量的变化
Table 3. Variation of slag content along the height of ingot
% 高度/mm Al2O3 TiO2 SiO2 MnO S1 S2 S3 S4 S1 S2 S3 S4 S1 S2 S3 S4 S1 S2 S3 S4 25 31.57 31.89 29.94 30.45 6.03 8.69 14.03 12.56 0.39 0.49 0.46 0.45 0.011 0.011 0.012 0.013 85 31.26 30.66 29.20 30.56 5.79 8.12 13.79 12.49 0.42 0.43 0.50 0.51 0.013 0.012 0.014 0.012 145 31.00 30.09 29.00 31.02 5.66 8.19 13.56 12.46 0.47 0.45 0.51 0.53 0.014 0.012 0.013 0.012 205 31.09 30.16 29.35 31.12 5.63 8.04 13.34 12.52 0.48 0.46 0.53 0.53 0.02 0.011 0.012 0.013 表 4 熔渣中可能存在的粒子结构,标准生成吉布斯自由能及作用浓度
Table 4. Possible units in slag and itsstandard Gibbs free energy of formation and action concentration
粒子类型 结构 编号 ${\Delta _r}G_i^\theta /({\text{J} } \cdot {\text{mo} }{ {\text{l} }^{ - 1} })$ Ni与Ki关系 质量作用浓度 简单离子 Ca2++O2− 1 ${N_1} = \dfrac{{2{n_1}}}{{\displaystyle\sum {{n_i}} }} = {N_{\rm CaO}}$ Mg2++O2− 2 ${N_2} = \dfrac{{2{n_2}}}{{\displaystyle\sum {{n_i}} }} = {N_{\rm MgO}}$ Fe2++O2− 3 ${N_3} = \dfrac{{2{n_3}}}{{\displaystyle\sum {{n_i}} }} = {N_{\rm FeO}}$ Mn2++O2− 4 ${N_4} = \dfrac{{2{n_4}}}{{\displaystyle\sum {{n_i}} }} = {N_{\rm MnO}}$ Ca2++2F− 5 ${N_5} = \dfrac{{3{n_5}}}{{\displaystyle\sum {{n_i}} }} = {N_{\rm Ca{F_2}}}$ 简单分子 SiO2 6 ${N_6} = \dfrac{{{n_6}}}{{\displaystyle\sum {{n_i}} }} = {N_{\rm Si{O_2}}}$ Al2O3 7 ${N_7} = \dfrac{{{n_7}}}{{\displaystyle\sum {{n_i}} }} = {N_{\rm A{l_2}{O_3}}}$ TiO2 8 ${N_8} = \dfrac{{{n_8}}}{{\displaystyle\sum {{n_i}} }} = {N_{\rm Ti{O_2}}}$ 复杂分子 3CaO·SiO2 c1 −118826−6.694T ${N_{c1}} = K_{c1}^\theta N_1^3{N_6}$ $ {N}_{c1}=\dfrac{{n}_{c1}}{{\displaystyle \sum {n}_{i}}}={N}_{\rm 3CaO·Si{O}_{2}} $ 3CaO·2SiO2 c2 −236814+9.623T ${N_{c2}} = K_{c2}^\theta N_1^3 N_6^2$ $ {N}_{c2}=\dfrac{{n}_{c2}}{{\displaystyle \sum {n}_{i}}}={N}_{\rm 3 CaO·2 Si{O}_{2}} $ 2CaO·SiO2 c3 −102090−24.267T ${N_{c3}} = K_{c3}^\theta N_1^2{N_6}$ $ {N}_{c3}=\dfrac{{n}_{c3}}{{\displaystyle \sum {n}_{i}}}={N}_{\rm 2 CaO·Si{O}_{2}} $ CaO·SiO2 c4 −21757−36.819T ${N_{c4}} = K_{c4}^\theta {N_1}{N_6}$ $ {N}_{c4}=\dfrac{{n}_{c4}}{{\displaystyle \sum {n}_{i}}}={N}_{\rm CaO·Si{O}_{2}} $ … … … … … 11CaO·7Al2O3·CaF2 c39 −228760−155.8T $ {N_{c{\text{39}}}} = K_{c{\text{39}}}^\theta N_{\text{1}}^{{\text{11}}}N_{\text{7}}^{\text{7}}{N_{\text{5}}} $ $ {N}_{c39}=\dfrac{{n}_{c39}}{{\displaystyle \sum {n}_{i}}}={N}_{\rm 11 CaO·7 A{l}_{2}{O}_{3}·Ca{F}_{2}} $ 3CaO·2SiO2·CaF2 c40 −255180−8.20T $ {N_{c{\text{40}}}} = K_{c{\text{40}}}^\theta N_{\text{1}}^{\text{3}}N_{\text{6}}^{\text{2}}{N_{\text{5}}} $ $ {N}_{c40}=\dfrac{{n}_{c40}}{{\displaystyle \sum {n}_{i}}}={N}_{\rm 3 CaO·2 Si{O}_{2}·Ca{F}_{2}} $ 表 5 文中使用的活度相互作用系数
$e_i^j$ Table 5. Activity interaction coefficient
$ {e}_{i}^{j} $ used in this study表 6 Al和Ti含量预测值
Table 6. Prediction Al and Ti contents by developed model
% 渣系 Al预测值 Ti预测值 S1 0.50 0.19 S2 0.32 0.38 S3&S4 0.18 0.84 -
[1] Scholz H, Biebricher U, Brückmann G, et al. ESR meets the requirements for big forgings[J]. Iron and Steel, 2013,48(10):82−87. (Scholz H, Biebricher U, Brückmann G, 等. 电渣重熔满足大型锻件的要求[J]. 钢铁, 2013,48(10):82−87.Scholz H, Biebricher U, Brückmann G, et al. ESR meets the requirements for big forgings[J]. Iron and Steel, 2013, 48(10): 82-87. [2] 李正邦. 电渣冶金的理论与实践[M]. 北京: 冶金工业出版社, 2010.Li Zhengbang. Electroslag metallurgy theory and practice[M]. Beijing: Metallurgical Industry Press, 2010. [3] Lin Jie, Yang Cheng, Wang Yuanming, et al. Deoxidation thermodynamics in electroslag remelting process[J]. Iron and Steel, 2019,54(9):44−49. (林杰, 杨成, 王远明, 等. 电渣重熔中的脱氧热力学[J]. 钢铁, 2019,54(9):44−49.Lin Jie, Yang Cheng, Wang Yuanming, et al. Deoxidation thermodynamics in electroslag remelting process[J]. Iron and Steel, 2019, 54 (9): 44-49. [4] Yin Bin, Li Wanming, Wu Shaopeng, et al. Thermodynamic analysis of Al and Ti element loss in electroslag remelting Inconel718 superalloy[J]. Iron and Steel, 2019,54(5):86−94. (尹彬, 李万明, 吴少鹏, 等. Inconel718高温合金电渣重熔铝钛元素烧损热力学分析[J]. 钢铁, 2019,54(5):86−94.Yin Bin, Li Wanming, Wu Shaopeng, et al. Thermodynamic analysis of Al and Ti element loss in electroslag remelting Inconel718 superalloy[J]. Iron and Steel, 2019, 54(5): 86-94. [5] Li Xing, Geng Xin, Jiang Zhouhua, et al. Influences of slag system on metallurgical quality for high temperature alloy by electroslag remelting[J]. Iron and Steel, 2015,50(9):41−46. (李星, 耿鑫, 姜周华, 等. 电渣重熔高温合金渣系对冶金质量的影响[J]. 钢铁, 2015,50(9):41−46.Li Xing, Geng Xin, Jiang Zhouhua, et al. Influences of slag system on metallurgical quality for high temperature alloy by electroslag remelting[J]. Iron and Steel, 2015, 50(9): 41-46. [6] Pateisky G, Biele H, Fleischer H J. The reactions of titanium and silicon with Al2O3−CaO−CaF2 slags in the ESR process[J]. Journal of Vacuum Science and Technology, 1972,9(6):1318−1321. doi: 10.1116/1.1317029 [7] Duan Shengchao, Guo Hanjie, Shi Xiao, et al. Thermodynamic analysis of smelting of Inconel718 suprealloy during electroslag remelting process[J]. Chinese Journal of Engineering, 2018,40(S1):53−64. (段生朝, 郭汉杰, 石骁, 等. Inconel718高温合金电渣重熔热力学分析[J]. 工程科学学报, 2018,40(S1):53−64.Duan Shengchao, Guo Hanjie, Shi Xiao, et al. Thermodynamic analysis of smelting of Inconel 718 suprealloy during electroslag remelting process[J]. Chinese Journal of Engineering, 2018, 40(S1): 53-64. [8] Jiang Zhouhua, Hou Dong, Dong Yanwu, et al. Effect of slag on titanium, silicon, aluminum content in superalloy during electroslag remelting[J]. Metallurgical and Materials Transitions B, 2016,47(2):1465−1474. doi: 10.1007/s11663-015-0530-8 [9] Li Shijian, Cheng Guoguang, Yang Liang, et al. A thermodynamic model to design the equilibrium slag compositions during electroslag remelting process: description and verification[J]. ISIJ International, 2017,57(4):713−722. doi: 10.2355/isijinternational.ISIJINT-2016-655 [10] Hou Dong, Jiang Zhouhua, Dong Yanwu, et al. Effect of slag composition on the oxidation kinetics of alloying elements during electroslag remelting of stainless steel: Part−2 control of titanium and aluminum content[J]. ISIJ International, 2017,57(8):1410−1419. doi: 10.2355/isijinternational.ISIJINT-2017-148 [11] Hou Dong, Jiang Zhouhua, Dong Yanwu, et al. Effect of slag composition on the oxidation kinetics of alloying elements during electroslag remelting of stainless steel: Part−1 mass−transfer model[J]. ISIJ International, 2017,57(8):1400−1409. doi: 10.2355/isijinternational.ISIJINT-2017-147 [12] Shi Chengbin, Zheng Dingli, Shin Seungho, et al. Effect of TiO2 on the viscosity and structure of low-fluoride slag used for electroslag remelting of Ti-containing steels[J]. Int. J. Miner. Metall. Mater., 2017,24(1):18−24. doi: 10.1007/s12613-017-1374-9 [13] 侯栋. 电渣重熔含铝钛不锈钢的冶金反应和成分控制研究[D]. 沈阳: 东北大学, 2017.Hou Dong. Metallurgical reaction and alloying element control during electroslag remelting of stainless steel containing aluminum and titainum[D]. Shenyang: Northeastern University, 2017. [14] Yang Xueming, Duan Jianping, Shi Chengbin, et al. A thermodynamic model of phosphorus distribution ratio between CaO-SiO2-MgO-FeO-Fe2O3-MnO-Al2O3-P2O5 slags and molten steel during a top–bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory[J]. Metallurgical and Materials Transitions B, 2011,42(4):738−770. doi: 10.1007/s11663-011-9491-8 [15] Zhang Bo, Jiang Maofa, Qi Jie, et al. Activity calculation model for a slag system consisting of CaO-SiO2-Al2O3-FeO-CaF2-La2O3-Nb2O5-TiO2[J]. Journal of Northeastern University, 2011,32(4):524−528. (张波, 姜茂发, 亓捷, 等. CaO-SiO2-Al2O3-FeO-CaF2-La2O3-Nb2O5-TiO2渣系的活度计算模型[J]. 东北大学学报, 2011,32(4):524−528.Zhang Bo, Jiang Maofa, Qi Jie, et al. Activity calculation model for a slag system consisting of CaO-SiO2-Al2O3-FeO-CaF2-La2O3-Nb2O5-TiO2[J]. Journal of Northeastern University, 2011, 32(4): 524-528. [16] Duan Shenchao, Li Chuang, Guo Xiaolong, et al. A thermodynamic model for calculation manganese distribution ratio between CaO-SiO2-MgO-FeO-MnO-Al2O3-TiO2-CaF2 ironmaking slags and carbon saturated hot metal based on the IMCT[J]. Irongmaking and Steelmaking, 2017,32(4):655−664. [17] Suito Hideaki, Inoue Ryo. Thermodynamics on control of inclusions composition in ultraclean steels[J]. ISIJ International, 1996,36(5):528−536. doi: 10.2355/isijinternational.36.528 [18] Sigworth G K, Elliott J F. The thermodynamics of liquid dilute iron alloys[J]. Metal Science, 1974,8:289−310. doi: 10.1016/0036-9748(74)90253-1 [19] Andrey Karasev, Hideaki Suito. Quantitative evaluation of inclusion in deoxidation of Fe−10 mass pct Ni alloy with Si, Ti, Al, Zr, and Ce[J]. Metallurgical and Materials Transactions B, 1999,30(2):249−257. doi: 10.1007/s11663-999-0054-1 [20] Mikine Kishi, Ryo Inoue, Hideaki Suito. Thermodynamics of oxygen and nitrogen in liquid Fe−20%Cr alloy equilibrated with titania−based slags[J]. ISIJ International, 1994,34(11):859−867. doi: 10.2355/isijinternational.34.859 -






 下载:
下载: