Effect of V doping on electrochemical properties of LiNi1/3Co1/3Mn1/3O2 as cathode material for lithium-ion battery
-
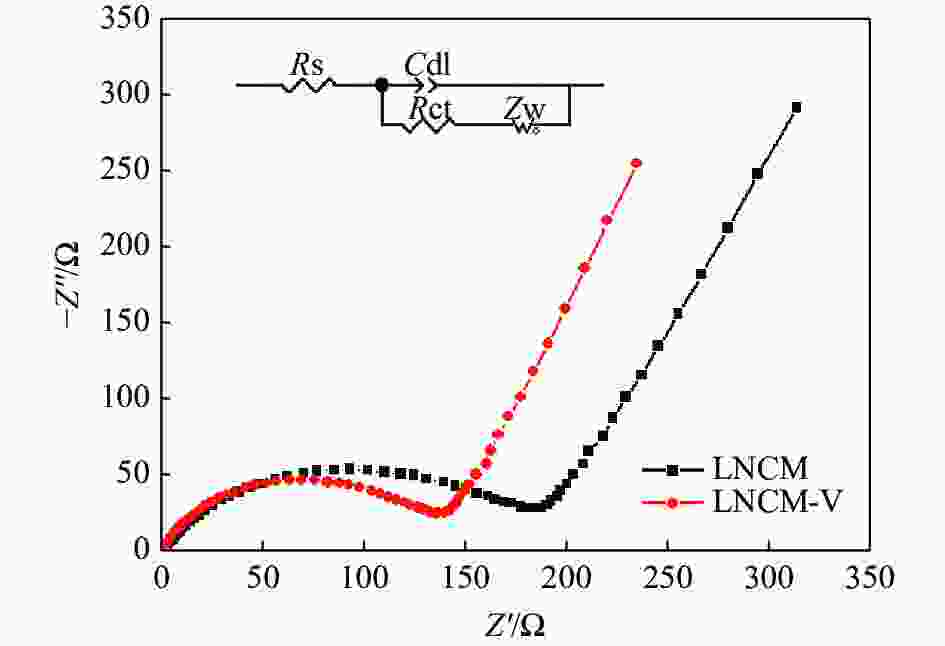
摘要: LiNi1/3Co1/3Mn1/3O2 (LNCM)因其高比容量等优点在动力电池领域受到广泛关注。然而,较差的循环性能和严重的安全问题限制了其应用前景。离子掺杂是提高材料电化学性能的有效方法之一。采用溶胶-凝胶法,以NH4VO3为钒源,成功制备了钒掺杂LiNi1/3-xCo1/3Mn1/3VxO2正极材料。结果表明:LiNi1/3-0.02Co1/3Mn1/3V0.02O2 (LNCM-V)电极材料表现出优秀的储锂性能(0.5 C时, 80次循环后,放电比容量为169 mAh/g)。通过V5+替代LiNi1/3Co1/3Mn1/3O2正极材料中部分Ni2+离子,有效地降低了Li+/Ni2+离子混排,稳定了正极材料的晶体结构,Li+离子的扩散系数得到增加,使Li+脱嵌过程中LNCM-V材料结构变得更加稳定。
-
关键词:
- 锂离子电池 /
- 正极材料 /
- LiNi1/3Co1/3Mn1/3O2 /
- 钒掺杂 /
- 电化学性能
Abstract: LiNi1/3Co1/3Mn1/3O2 (LNCM) cathode material with high specific capacity has received extensive attention in power batteries. However, the serious safety issues and poor cycle performance limit its application prospect. Doping is one of the effective methods to boost the electrochemical performance of electrode materials. Vanadium-doped LiNi1/3-xCo1/3Mn1/3VxO2 cathode material was successfully prepared by the sol-gel method using NH4VO3 as vanadium source. The research results show that by replacing some Ni2+ ions in the LiNi1/3Co1/3Mn1/3O2 cathode material by V5+, the cation mixing is effectively reduced, the crystal structure of the cathode material is stabilized, and the diffusion coefficient of Li+ in the lattice increases. And the LiNi1/3-0.02Co1/3Mn1/3V0.02O2 (LNCM-V) electrode material exhibits excellent lithium storage performance (the discharge capacity is 169 mAh/g at 0.5 C after 80 cycles).-
Key words:
- lithium ion battery /
- cathode material /
- LiNi1/3Co1/3Mn1/3O2 /
- V doping /
- electrochemical properties
-
图 3 (a) LNCM和LNCM-V样品的循环性能,(b) LNCM-V样品的充放电曲线,(c) LNCM和LNCM-V样品的库仑效率,(d) LNCM和LNCM-V样品的倍率性能
Figure 3. (a) Cycle performances of the LNCM and LNCM-V samples, (b) Charge/discharge profiles of the LNCM-V sample, (c) Coulombic efficiency of the LNCM and LNCM-V samples, (d) Rate performance of the LNCM and LNCM-V samples
表 1 LNCM和 LNCM-V样品的晶胞参数
Table 1. Lattice parameters of the LNCM and LNCM-V samples
a/nm c /nm R(I003/I104) LNCM 0.28561 1.4223 1.22 LNCM-V 0.28593 1.4235 1.36 -
[1] Aida T, Tsutsui Y, Kanada S, et al. Ammonium tungstate modified Li-rich Li1+xNi0.35Co0.35Mn0.30O2 to improve rate capability and productivity of lithium-ion batteries[J]. Journal of Solid State Electrochemistry, 2017,21:2047−2054. doi: 10.1007/s10008-017-3586-3 [2] Hy S, Liu H, Zhang M, et al. Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries[J]. Energy Environmental Science, 2016,9:1931−1954. doi: 10.1039/C5EE03573B [3] Chen Y, Wang G X, Konstantinov K, et al. Synthesis and characterization of LiCoxMnyNi1-x-yO2 as a cathode material for secondary lithium batteries[J]. Journal of Power Sources, 2003,119-121:184−188. [4] Sun Y K, Myung S T, Park B C, et al. High-energy cathode material for long-life and safe lithium batteries[J]. Nature Materials, 2009,8:320−324. doi: 10.1038/nmat2418 [5] Yu Y, Luo Y F, Wu H C, et al. Ultrastretchable carbon nanotube composite electrodes for flexible lithium-ion batteries[J]. Nanoscale, 2018,10:19972−19978. doi: 10.1039/C8NR05241G [6] Zheng H, Chen X, Yang Y, et al. Self-assembled LiNi1/3Co1/3Mn1/3O2 nanosheet cathode with high electrochemical performance[J]. ACS Applied Materials Interfaces, 2017,9:39560−39568. doi: 10.1021/acsami.7b10264 [7] Yoon C S, Park K J, Kim U H, et al. High-energy Ni rich Li[NixCoyMn1-x-y]O2 cathodes via compositional partitioning for next generation electric vehicles[J]. Chemistry of Materials, 2017,29:10436−10445. doi: 10.1021/acs.chemmater.7b04047 [8] Li L, Liu Q, Huang J J, et al. Synthesis and electrochemical properties of Zn-doping LiNi1/3Co1/3Mn1/3O2 cathode material for lithium-ion battery application[J]. Journal of Materials Science:Materials in Electronics, 2020,31:12409−12416. doi: 10.1007/s10854-020-03787-9 [9] Yang X Q, Tang Z F, Wang H Y, et al. Improving the electrochemical performance of LiNi0.5Co0.2Mn0.3O2 by double-layer coating with Li2TiO3 for lithium-ion batteries[J]. Ionics, 2016,22:2235−2238. doi: 10.1007/s11581-016-1792-0 [10] Yang X H, Zuo Z C, Wang H Y, et al. The contradiction between the half-cell and full-battery evaluations on the tungsten-coating LiNi0.5Co0.2Mn0.3O2 cathode[J]. Electrochimica Acta, 2015,180:604−609. doi: 10.1016/j.electacta.2015.08.150 [11] Yang D J, Li X J, Wu N N, et al. Effect of moisture content on the electrochemical performance of LiNi1/3Co1/3Mn1/3O2/graphite battery[J]. Electrochimica Acta, 2016,188:611−618. doi: 10.1016/j.electacta.2015.12.063 [12] Dianat A, Seriani N, Bobeth M, et al. Effects of Al-doping on the properties of Li–Mn–Ni–O cathode materials for Li-ion batteries: an ab initio study[J]. Journal of Materials Chemistry A, 2013,1:9273−9280. doi: 10.1039/c3ta11598d [13] Li H J, Chen G, Zhang B, et al. Advanced electrochemical performance of Li[Ni(1/3−x)FexCo1/3Mn1/3]O2 as cathode materials for lithium-ion battery[J]. Solid State Communications, 2008,146:115−120. doi: 10.1016/j.ssc.2008.02.006 [14] Chen Y H, Zhang J, Li Y, et al. Effects of doping high-valence transition metal (V, Nb and Zr) ions on the structure and electrochemical performance of LIB cathode material LiNi0.8Co0.1Mn0.1O2[J]. Physical Chemistry Chemical Physics, 2021,23:11528. doi: 10.1039/D1CP00426C [15] Zhu H L, Xie T, Chen Z Y, et al. The impact of vanadium substitution on the structure and electrochemical performance of LiNi0.5Co0.2Mn0.3O2[J]. Electrochimica Acta, 2014,135:77−85. doi: 10.1016/j.electacta.2014.04.183 [16] Hu Z Y, Wang L L, Luo Y Z, et al. Vanadium-doped LiNi1/3Co1/3Mn1/3O2 with decreased lithium/nickel disorder as high-rate and long-life lithium ion battery cathode[J]. Science Advanced Today, 2015,1:25218. [17] Yang C F, Zhang X S, Huang M Y, et al. Preparation and rate capability of carbon coated LiNi1/3Co1/3Mn1/3O2 as cathode material in lithium ion batteries[J]. ACS Applied Materials Interfaces, 2017,9:12408−12415. doi: 10.1021/acsami.6b16741 [18] Riekehr L, Liu J L, Schwarz B, et al. Effect of pristine nanostructure on first cycle electrochemical characteristics of lithium-rich lithium-nickel-cobalt-manganese-oxide cathode ceramics for lithium ion batteries[J]. Journal of Power Sources, 2016,306:135−147. doi: 10.1016/j.jpowsour.2015.11.082 [19] Meng X, Cao H, Hao J, et al. Sustainable preparation of LiNi1/3Co1/3Mn1/3O2-V2O5 cathode materials by recycling waste materials of spent lithium-ion battery and vanadium-bearing slag[J]. ACS Sustainable Chemistry Engineering, 2018,6:5797−5805. doi: 10.1021/acssuschemeng.7b03880 [20] Peng L L, Zhu Y, Khakoo U, et al. Self-assembled LiNi1/3Co1/3Mn1/3O2 nanosheet cathodes with tunable rate capability[J]. Nano Energy, 2015,17:36−42. doi: 10.1016/j.nanoen.2015.07.031 [21] Wu F, Wang M, Su Y, et al. Effect of TiO2-coating on the electrochemical performances of LiNi1/3Co1/3Mn1/3O2[J]. Journal of Power Sources, 2009,191:628−632. doi: 10.1016/j.jpowsour.2009.02.063 [22] Cabelguen P E, Peralta D, Cugnet M, et al. Impact of morphological changes of LiNi1/3Co1/3Mn1/3O2 on lithium-ion cathode performances[J]. Journal of Power Sources, 2017,346:13−23. doi: 10.1016/j.jpowsour.2017.02.025 -





 下载:
下载: