Effect of hydrothermal reaction time on electrochemical properties of (NH4)2V4O9 as cathode material for aqueous zinc ion batteries
-
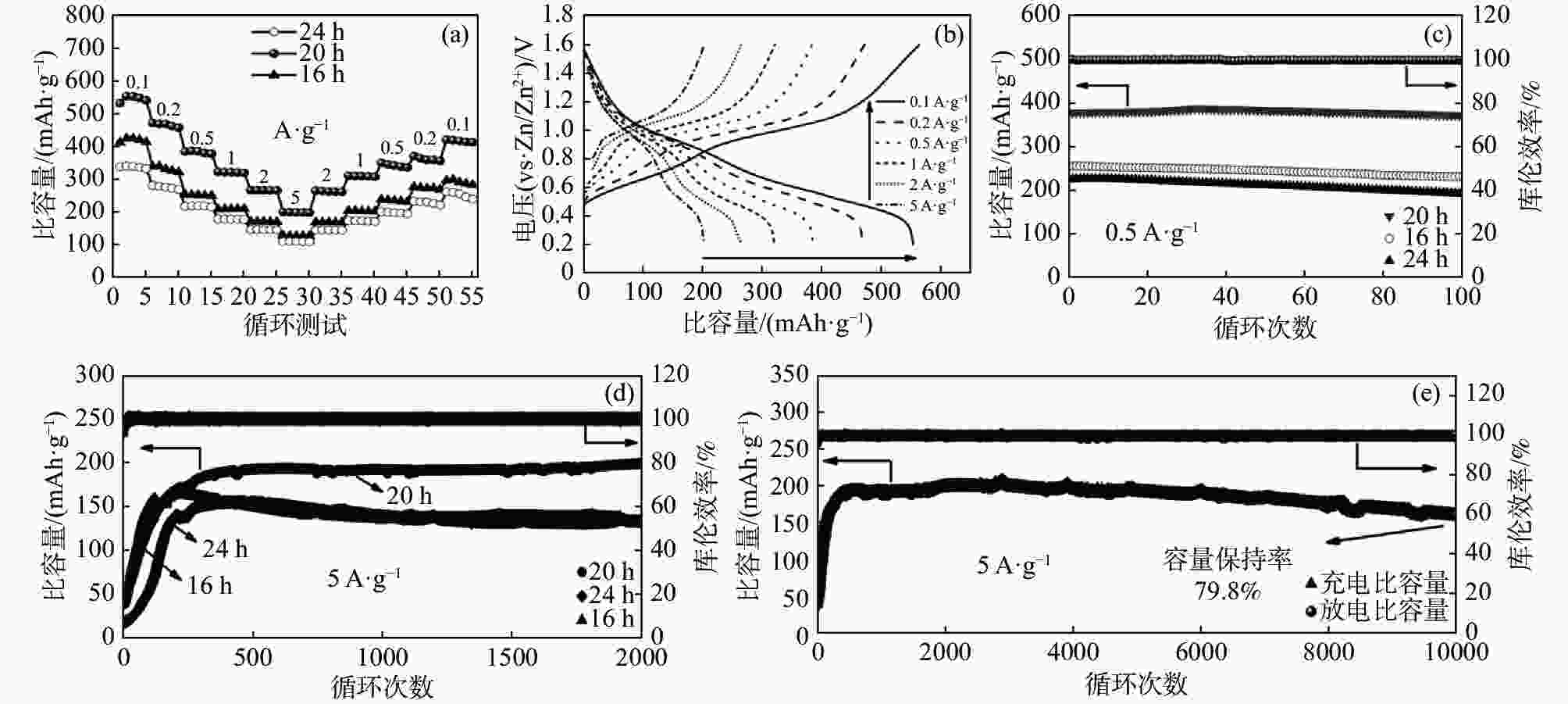
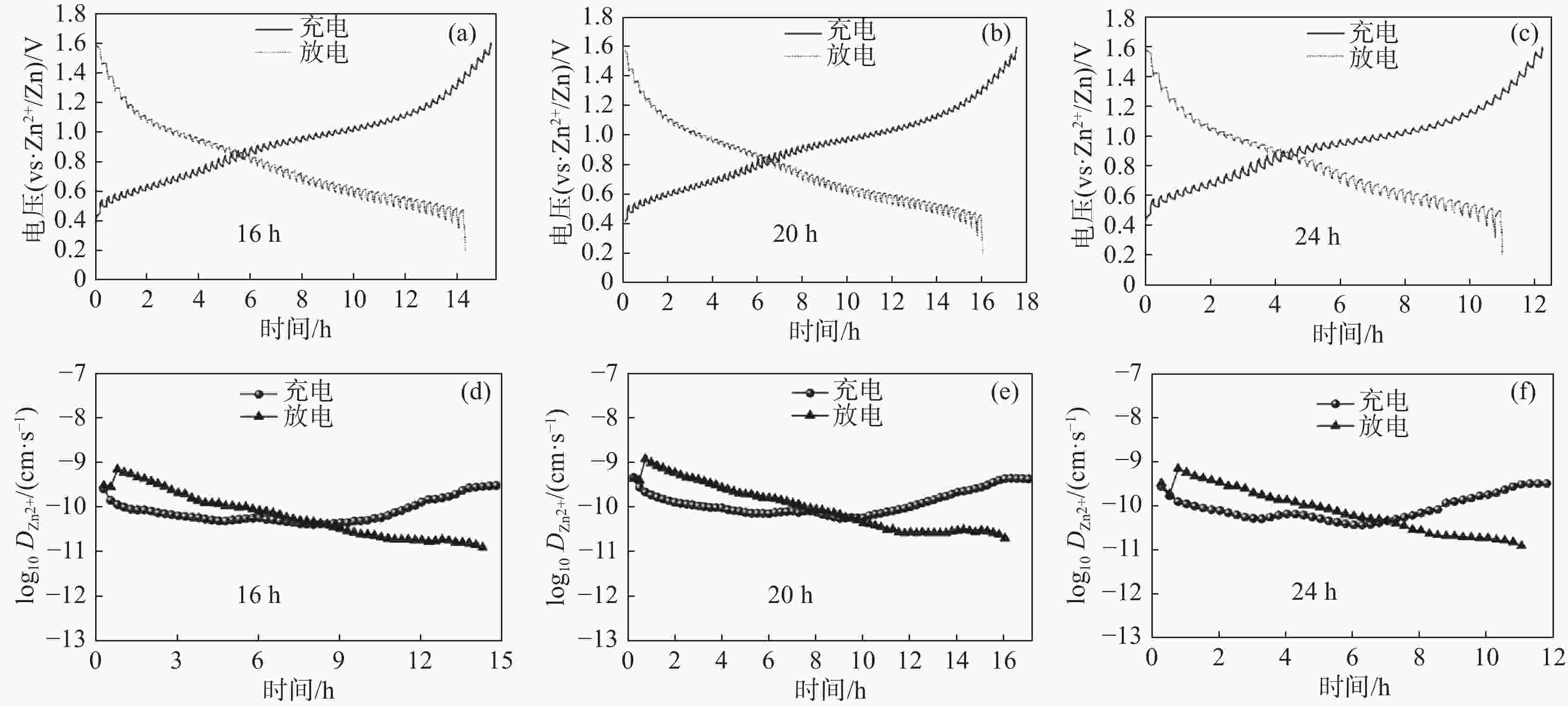
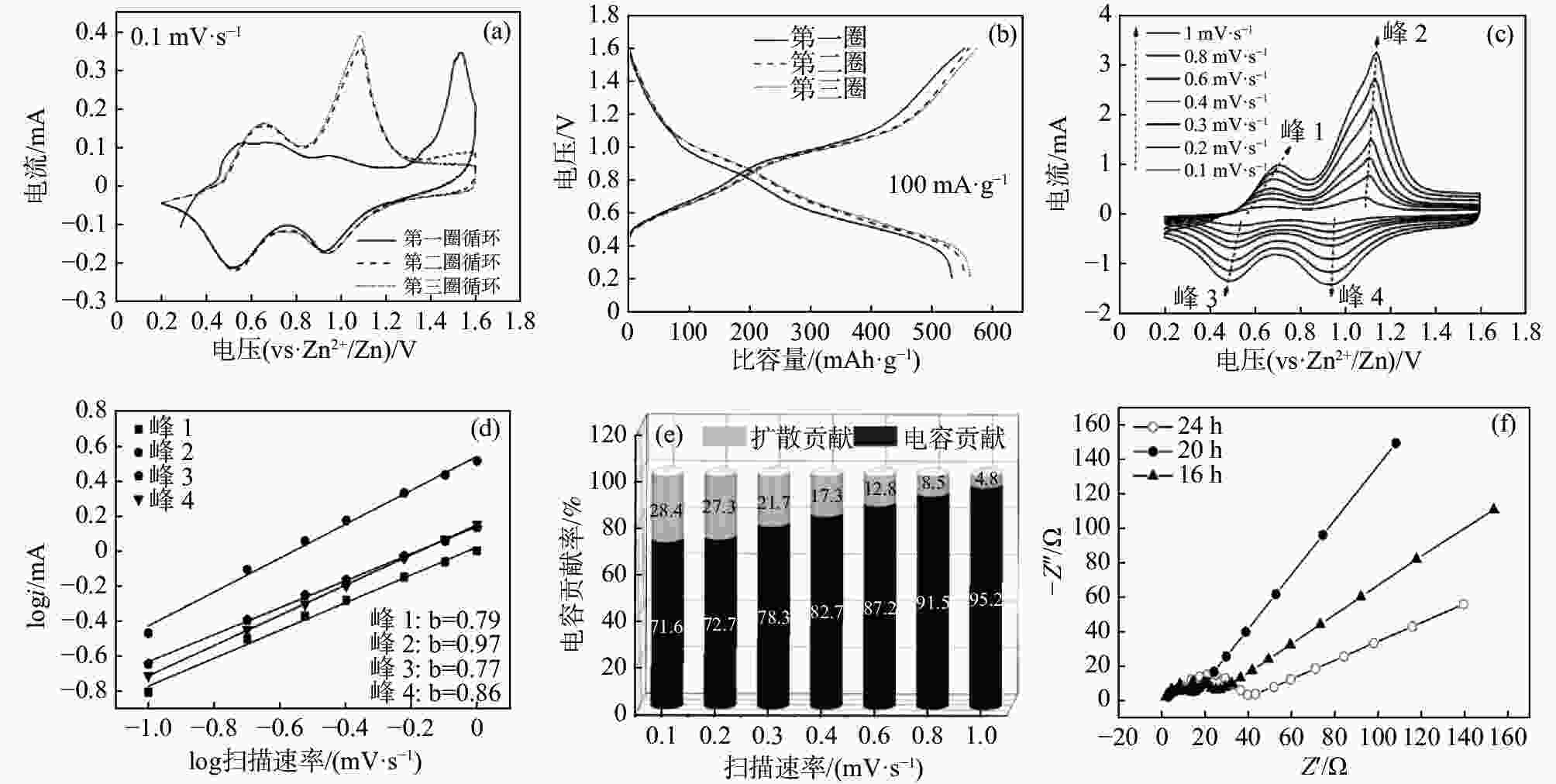
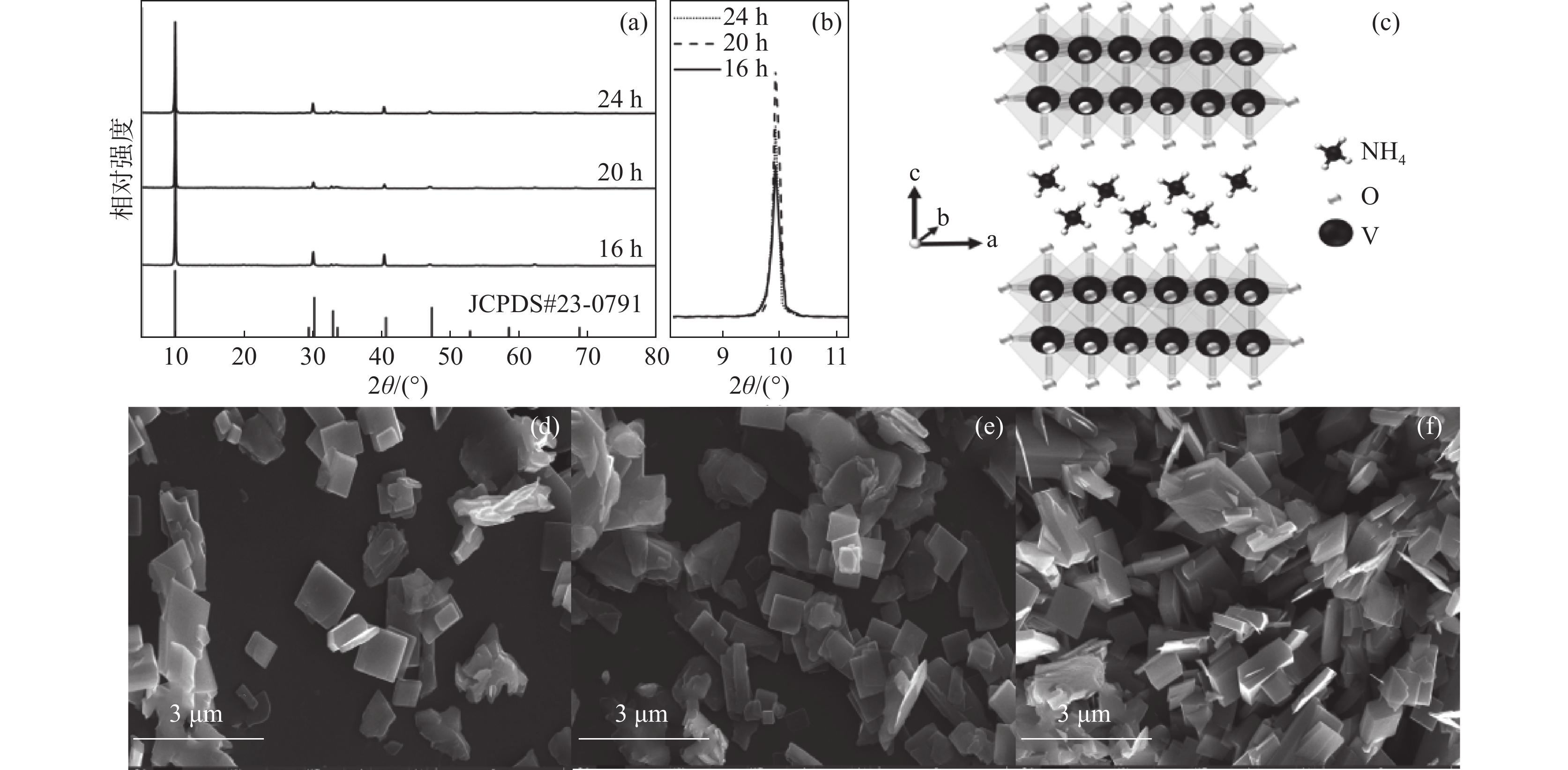
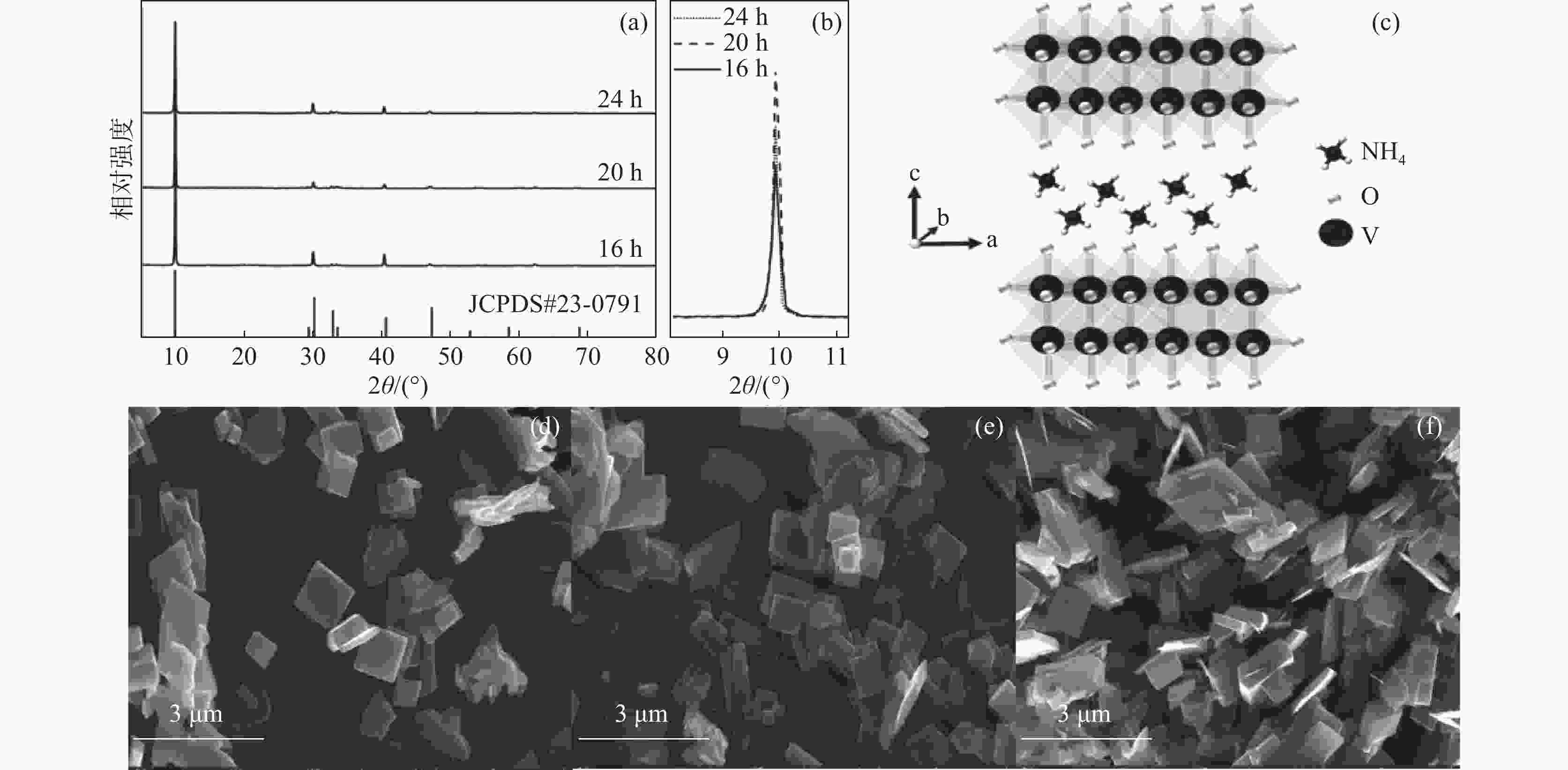
摘要: 以NH4VO3和C2H2O4·2H2O为原料,采用简易的一步水热法制备出水系锌离子电池正极材料(NH4)2V4O9。利用X射线衍射仪(XRD)、扫描电子显微镜(SEM)、恒流充放电(GCD)、恒流间歇滴定技术(GITT)、循环伏安法(CV)以及电化学阻抗谱(EIS)等表征测试手段,研究不同水热反应时间(16、20和24 h)对 (NH4)2V4O9结构、形貌及电化学性能的影响。研究结果表明:水热反应20 h合成的(NH4)2V4O9拥有最高的结晶度,并表现出优异的电极反应动力学特性以及最佳的倍率性能和循环稳定性,其在0.1、0.2、0.5、1、2 A/g和5 A/g的电流密度下分别提供了554.6、472.2、386.6、322.6、266.2 mAh/g和199.5 mAh/g的高放电比容量,并且在5 A/g的大电流密度下长循环10 000圈后仍可保持159.7 mAh/g的放电比容量,容量保持率高达80.1%。
-
关键词:
- 水系锌离子电池 /
- 钒基正极材料 /
- (NH4)2V4O9 /
- 水热反应时间 /
- 电化学性能
Abstract: (NH4)2V4O9 as cathode material for aqueous zinc ion batteries was prepared by a facile one-step hydrothermal method using NH4VO3 and C2H2O4·2H2O as raw materials. X-ray diffractometer (XRD), scanning electron microscope (SEM), galvanostatic charge-discharge (GCD), galvanostatic intermittent titration technique (GITT), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were used to investigate the effects of hydrothermal reaction time (16, 20 h and 24 h) on the structure, morphology and electrochemical properties of (NH4)2V4O9. The results show that (NH4)2V4O9 synthesized by hydrothermal reaction for 20 h has the highest crystallinity, electrode reaction kinetics, and rate capability as well as cyclic stability, delivering high discharge specific capacities of 554.6, 472.2, 386.6, 322.6, 266.2 and 199.5 mAh/g at current densities of 0.1, 0.2, 0.5, 1, 2 and 5 A/g, respectively. It can also maintain a discharge capacity of 159.7 mAh/g after 10 000 long-term cycles at a high current density of 5 A/g, showing a capacity retention of up to 80.1%. -
表 1 试验原料规格与厂家
Table 1. Specifications and manufacturers of raw materials
名称 规格 厂家 偏钒酸铵(NH4VO3) 分析纯 成都市科隆化学品有限公司 草酸(C2H2O4·H2O) 分析纯 成都市科隆化学品有限公司 导电碳黑 电池级 东莞市科路得实验器材科技有限公司 聚偏氟乙烯(PVDF) 电池级 东莞市科路得实验器材科技有限公司 N-甲基吡咯烷酮(NMP) 电池级 成都市科隆化学品有限公司 锌箔(Zn) 电池级 东莞市科路得实验器材科技有限公司 钛箔(Ti) 电池级 东莞市科路得实验器材科技有限公司 三氟甲烷磺酸锌(Zn(CF3SO3)2) 分析纯 上海麦克林生化科技有限公司 玻璃纤维滤膜 GF/D 英国Whatman公司 -
[1] Randau S, Weber D A, Kötz O, et al. Benchmarking the performance of all-solid-state lithium batteries[J]. Nature Energy, 2020,5(3):259−270. doi: 10.1038/s41560-020-0565-1 [2] Zubi G, Dufo-López R, Carvalho M, et al. The lithium-ion battery: State of the art and future perspectives[J]. Renewable and Sustainable Energy Reviews, 2018,89:292−308. doi: 10.1016/j.rser.2018.03.002 [3] Essl C, Golubkov A W, Gasser E, et al. Comprehensive hazard analysis of failing automotive lithium-ion batteries in overtemperature experiments[J]. Batteries, 2020,6(2):30. doi: 10.3390/batteries6020030 [4] Li Xin, Qi Shihan, Zhang Wenchao, et al. Recent progress on FeS2 as anodes for metal-ion batteries[J]. Rare Metals, 2020,39(11):1239−1255. doi: 10.1007/s12598-020-01492-4 [5] Ming Jun, Guo Jing, Xia Chuan, et al. Zinc-ion batteries: Materials, mechanisms, and application[J]. Materials Science and Engineering:R:Reports, 2019,135:58−84. doi: 10.1016/j.mser.2018.10.002 [6] Alfaruqi M H, Mathew V, Song J, et al. Electrochmical zinc intercalation in lithium vanadium oxide: A high-capacity zinc-ion battery cathode[J]. Chemistry of Materials, 2017,29(4):1684−1694. doi: 10.1021/acs.chemmater.6b05092 [7] Zhu Chuyu, Fang Guozhao, Liang Shuquan, et al. Electrochemically induced cationic defect in MnO intercalation cathode for aqueous zinc-ion battery[J]. Energy Storage Materials, 2020,24:394−401. doi: 10.1016/j.ensm.2019.07.030 [8] Zhou Hua, Song Yongchang, Liu Jin, et al. Progess of vanadium-based electrode materal in energy storage[J]. Iron Steel Vanadium Titanium, 2022,43(2):73−80. (周华, 宋永昌, 刘进, 等. 钒基电极材料在储能领域的研究进展[J]. 钢铁钒钛, 2022,43(2):73−80. doi: 10.7513/j.issn.1004-7638.2022.02.012Zhou Hua, Song Yongchang, Liu Jin, et al. Progess of vanadium-based electrode materal in energy storage[J]. Iron Steel Vanadium Titanium, 2022, 43(2): 73-80. doi: 10.7513/j.issn.1004-7638.2022.02.012 [9] Yi Haocong, Qin Runzhi, Ding Shouxiang, et al. Structure and properties of prussian blue analogues in energy storage and conversion applications[J]. Advanced Functional Materials, 2021,31(6):2006970. doi: 10.1002/adfm.202006970 [10] Wang Fei, Hu Enyuan, Sun Wei, et al. A rechargeable aqueous Zn2+-battery with high power density and a long cycle-life[J]. Energy & Environmental Science, 2018,11(11):3168−3175. [11] Soundharrajan V, Sambandam B, Kim S, et al. Na2V6O16· 3H2O barnesite nanorod: an ope doo to display a stable and high energy for aqueous rechargeable Zn-ion batteies as cathodes[J]. Nano Letters, 2018,18(4):2402−2410. doi: 10.1021/acs.nanolett.7b05403 [12] Rehman Lashari N, Zhao M, Zheng Q, et al. Enhanced rate capability and cycling stability of novel ammonium vanadate materials used in aqueous Li-ion batteries[J]. Energy & Fuels, 2021,35(5):4570−4576. [13] Kim S, Soundharrajan V, Kim S, et al. Microwave-assisted rapid syntesis of NH4V4O10 layered oxide: A high energy cathode for aqueous rechargeable zinc ion batteries[J]. Nanomaterials, 2021,11(8):1905. doi: 10.3390/nano11081905 [14] Jiang Hanmei, Zhang Yifu, Xu Lei, et al. Fabrication of (NH4)2V3O8 nanparticles encapslated in amorphous carbonfor high capacity electrodes in aqueous zinc ion batteries[J]. Chemical Engineering Journal, 2020,382:122844. doi: 10.1016/j.cej.2019.122844 [15] Jiang Yingchang, Wu Zeyi, Ye Fei, et al. Spontaneous knitting behavior of 6.7-nm thin (NH4)0.38V2O5 nanoribbons for binder-free zinc-ion batteries[J]. Energy Storage Materials, 2021,42:286−294. doi: 10.1016/j.ensm.2021.07.045 [16] Zhang Yifu, Jiang Hanmei, Xu Lei, et al. Ammonium vanadium oxide [(NH4)2V4O9] shets for high capacity electrodes in aqueous zinc ion batteries[J]. ACS Applied Energy Materials, 2019,2(11):7861−7869. doi: 10.1021/acsaem.9b01299 [17] Cui Fuhan, Hu Fang, Yu Xin, et al. In-situ tuning the NH4+ extraction in (NH4)2V4O9 nano sheets towards high performance aqueous zinc ion batteries[J]. Journal of Power Sources, 2021,492:229629. doi: 10.1016/j.jpowsour.2021.229629 [18] Zhan Dan, Yang Fan, Zhang Qinggang, et al. Effect of solid-state reaction temperature on electrochemical performance of LiMn2O4 submicro-rods as cathode material for Li-ion battery by using γ-MnOOH submicro-rods as self-template[J]. Electrochimica Acta, 2014,129:364−372. doi: 10.1016/j.electacta.2014.02.141 [19] Zeng Jing, Zhang Zhonghua, Guo Xiaosong, et al. A conjugated polyaniline and water co-intercalation strategy boosting zinc-ion storage performances for rose-like vanadium oxide architectures[J]. Journal of Materials Chemistry A, 2019,7(37):21079−21084. doi: 10.1039/C9TA08086D [20] Hu Fang, Xie Di, Cui Fuhan, et al. Synthesis and electrochemical performance of NaV3O8 nanobelts for Li/Na-ion batteries and aqueous zinc-ion batteries[J]. RSC advances, 2019,9(36):20549−20556. doi: 10.1039/C9RA04339J [21] Zhou Kai, Wang Shuwei, Zhang Shichao, et al. Investigating the inceased-capacity mechanism of porous carbonmaterials in lithium-ion batteries[J]. Journal of Materials Chemistry A, 2020,8(28):14031−14042. doi: 10.1039/D0TA04054A [22] Fleischmann S, Mitchell J B, Wang R, et al. Pseudocapacitance: from fundamentall understanding to high power energy storage materials[J]. Chemical Reviews, 2020,120(14):6738−6782. doi: 10.1021/acs.chemrev.0c00170 [23] Zhu Aiyue, Wu Tao, Huang Kevin. NaCa0.6V6O16·3H2O as an ultra‐stable cahode for Zn‐ion batteries: the roles of pre‐inserted dual‐cations and structural water in V3O8 layer[J]. Advanced Energy Materials, 2019,9(38):1901968. doi: 10.1002/aenm.201901968 [24] Guo Chenxiao, Liu Yang, Wang Liqiu. Aquinoneamine polymer and its application as cathode material in aqueous zinc ion batteries[J]. Journal of Yanshan University, 2022,46(1):82−89. (郭晨晓, 刘洋, 王丽秋. 一种醌胺聚合物正极材料在水系锌离子电池中的应用[J]. 燕山大学学报, 2022,46(1):82−89. doi: 10.3969/j.issn.1007-791X.2022.01.010Guo Chenxiao, Liu Yang, Wang Liqiu. Aquinoneamine polymer and its application as cathode material in aqueous zinc ion batteries[J]. Journal of Yanshan University, 2022, 46(1): 82-89. doi: 10.3969/j.issn.1007-791X.2022.01.010 [25] Esparcia Jr E A, Chae M S, Ocon J D, et al. Ammonium vanadium bronze (NH4V4O10) as a high-capacity cathode material for nonaqueous magnesium-ion batteries[J]. Chemistry of Materials, 2018,30(11):3690−3696. doi: 10.1021/acs.chemmater.8b00462 [26] Zhang Leyuan, Chen Liang, Zhou Xufeng, et al. Towards high‐voltage aqeous metal‐ion batteries beyond 1.5 V: the zinc/zinc hexacyanoferrate system[J]. Advanced Energy Materials, 2015,5(2):1400930. doi: 10.1002/aenm.201400930 [27] Pan Zikang, Ru Qiang, Zheng Minghui, et al. Constrution of hierarchical flower‐shaped (NH4)2V3O8/rGO with enhanced zinc storage performance[J]. Chem. Electro. Chem., 2021,8(23):4618−4624. -




 下载:
下载: