Development of activity calculation model for the CaO-Al2O3-MgO-SiO2-Ce2O3 slag system
-
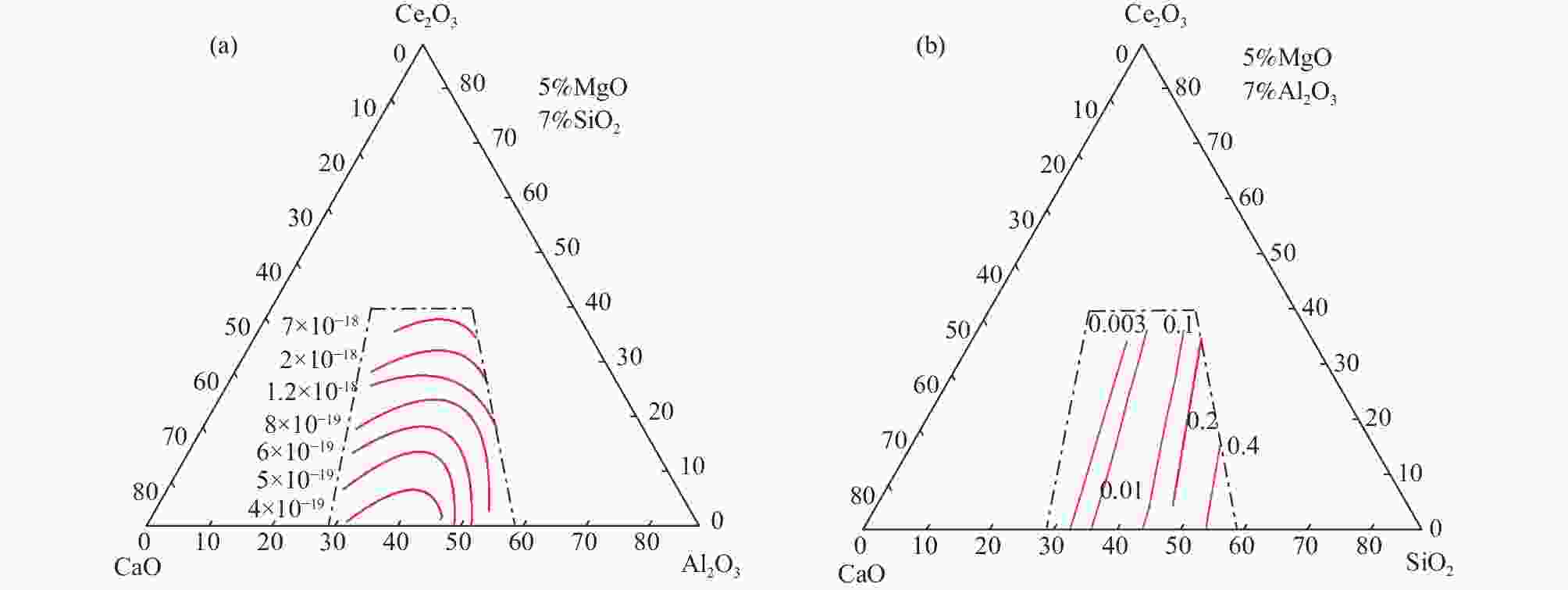
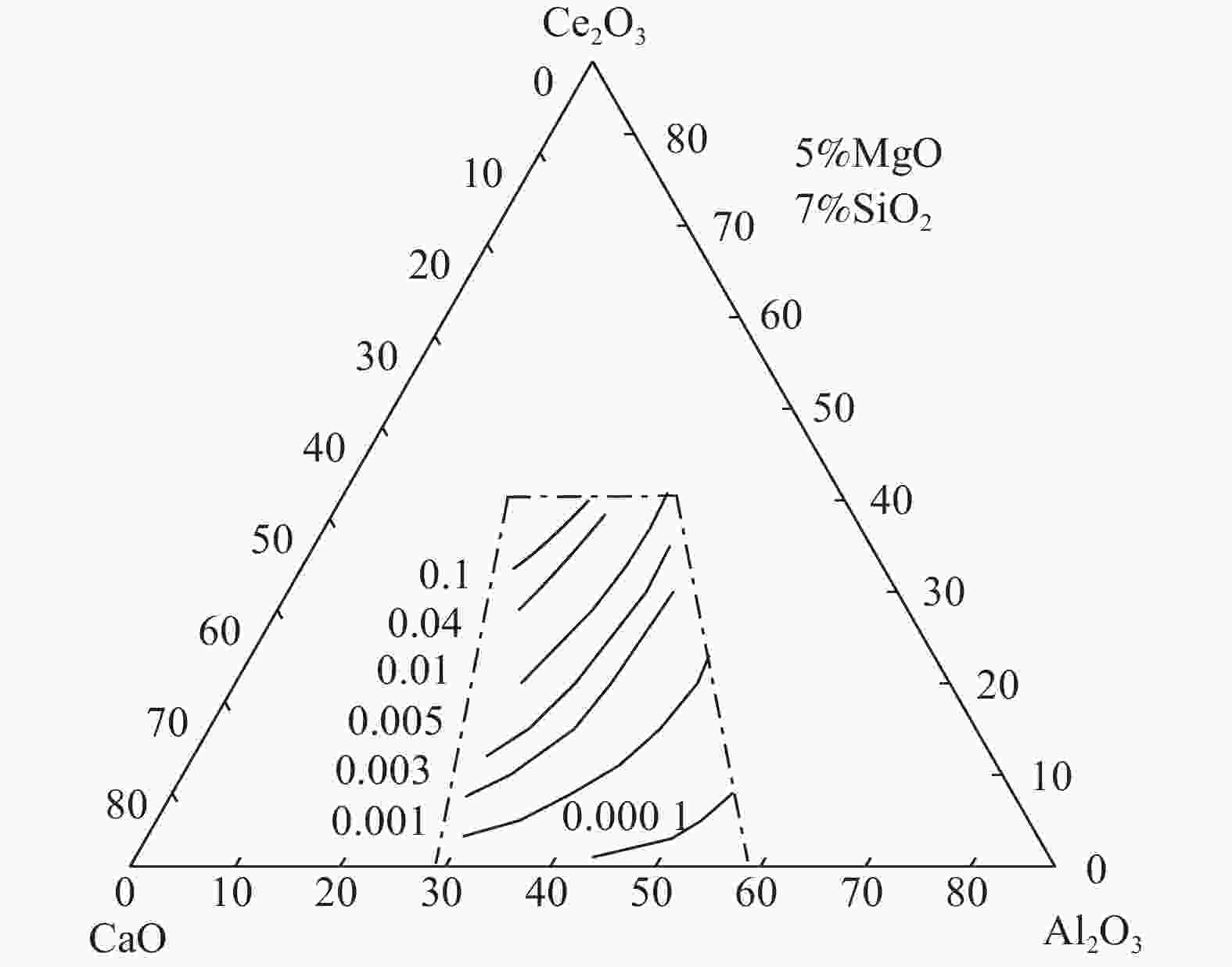
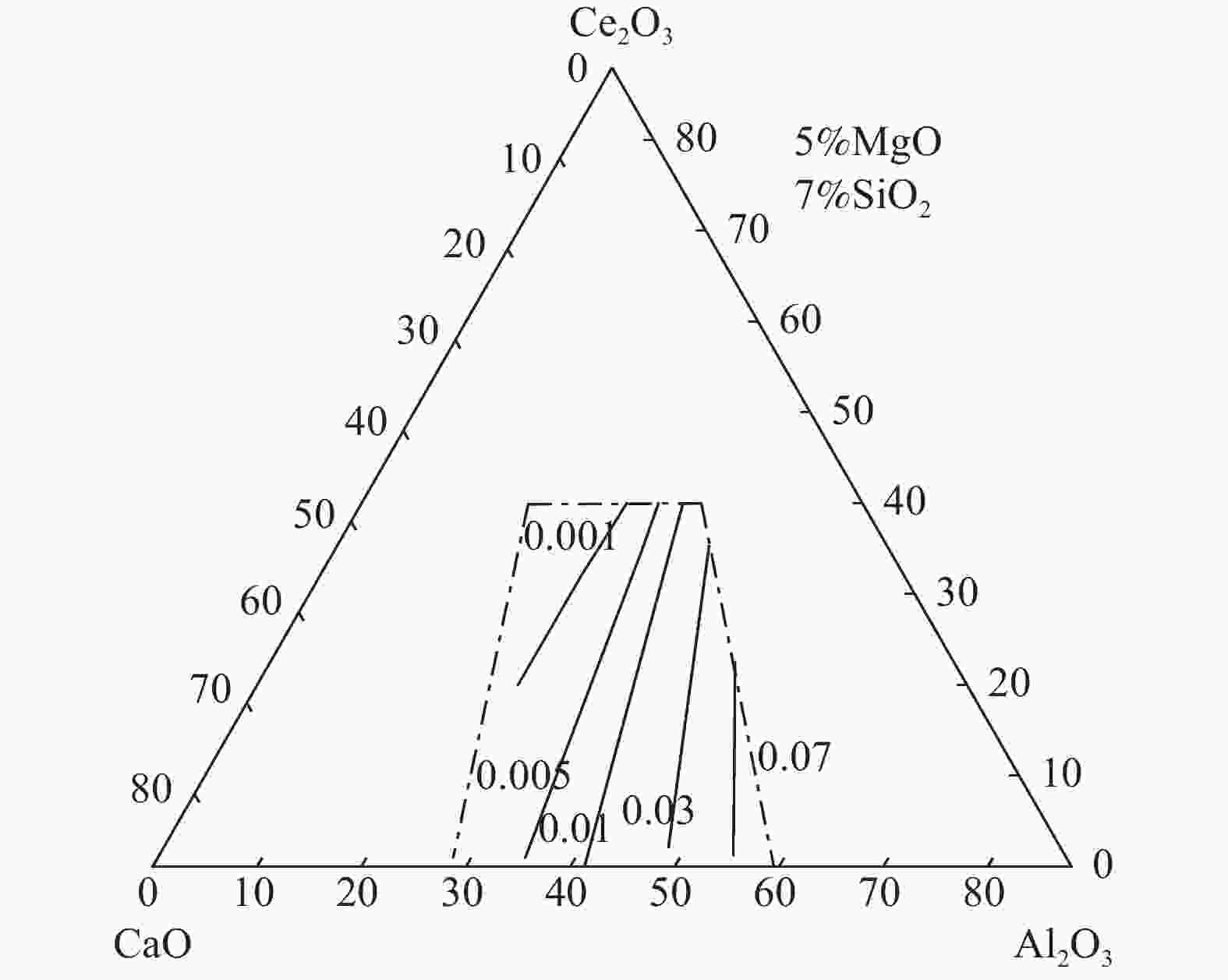
摘要: 稀土钢冶炼过程中,含稀土钢液与熔渣间存在强烈的渣金反应,导致钢中溶解态稀土含量波动严重,影响其在钢中的作用效果。然而,由于含稀土渣系热力学数据的缺失,限制了相关研究工作的开展。依据分子离子共存理论,建立了CaO-Al2O3-MgO-SiO2-Ce2O3渣系的活度计算模型,并绘制组元的等活度线图,讨论w(CaO)/w(Al2O3)、w(Ce2O3)等成分变化对渣系中各组元活度的影响。计算结果表明,CaO-Al2O3基精炼渣的SiO2活度范围(4×10−19~7×10−18)远小于CaO-SiO2基精炼渣的活度范围(0.003~0.4),即对于稀土钢而言,CaO-Al2O3基精炼渣更为适用;对于CaO-Al2O3基精炼渣,提高熔渣的w(CaO)/w(Al2O3)和w(Ce2O3)可增加Ce2O3活度、减小Al2O3活度,能够减弱含稀土钢液与熔渣中Al2O3间的反应。Abstract: During smelting steel containing rare earth elements, there exists strong reaction between the molten steel and the slag, which may result in significant content fluctuation of dissolved rare earth and then reduce its effect in steel. However, the lack of thermodynamic data of rare earth-containing slag system limits the development of related research work. In this paper, based on the ion and molecule coexistence theory, an activity calculation model of the CaO-Al2O3-MgO-SiO2-Ce2O3 slag system has been established, which can be used to construct the iso-activity diagram and investigate w(CaO)/w(Al2O3), w(Ce2O3) and other composition changes on the activity of each component of the slag system. The results show that the SiO2 activity range (4×10−19~7×10−18) of CaO-Al2O3-based refining slag is much smaller than that of CaO-SiO2-based refining slag (0.003~0.4), that is, CaO-Al2O3-based slag is more suitable for rare earth steel. For CaO-Al2O3-based refining slag, increasing the w(CaO)/w(Al2O3) and w(Ce2O3) of the slag can increase the activity of Ce2O3 and decrease the activity of Al2O3 , which may restrain the reaction between the rare earth-containing molten steel and Al2O3 in the slag.
-
Key words:
- rare earth steel /
- slag system /
- activity /
- ion and molecule coexistence theory
-
表 1 CaO-Al2O3-MgO-SiO2-Ce2O3结构单元
Table 1. Structural units of CaO-Al2O3-MgO-SiO2-Ce2O3
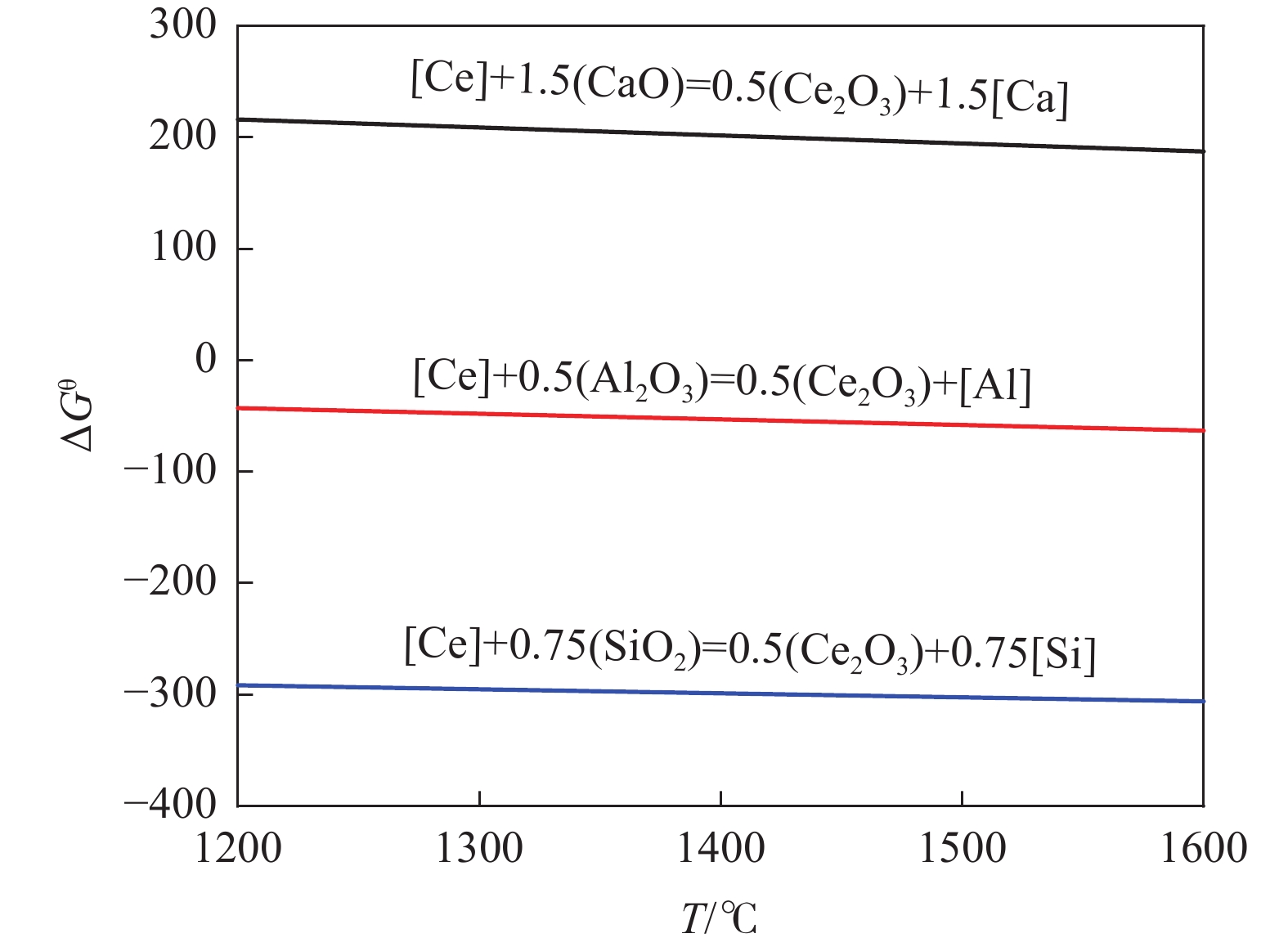
体系 结构单元 基本结构单元 Ca2+、Ce3+、Mg2+、Al2O3、SiO2 Al2O3-CaO CaO∙Al2O3、3CaO∙Al2O3、CaO∙2Al2O3、CaO∙6Al2O3 Al2O3-Ce2O3 Ce2O3·Al2O3、Ce2O3·11Al2O3 Al2O3-SiO2 3Al2O3·2SiO2 Al2O3-MgO MgO·Al2O3 SiO2-CaO CaO·SiO2、3CaO·2SiO2、2CaO·SiO2、3CaO·SiO2 SiO2-Ce2O3 Ce2O3·SiO2、Ce2O3·2SiO2、Ce4.67Si3O13 SiO2-MgO 2MgO·SiO2、MgO·SiO2 CaO-Al2O3-SiO2 2CaO·Al2O3·SiO2、CaO·Al2O3·2SiO2、CaO·Al2O3·SiO2、3CaO·Al2O3·3SiO2 CaO-SiO2-MgO CaO·MgO·2SiO2、CaO·MgO·SiO2、2CaO·MgO·2SiO2、3CaO·MgO·2SiO2 MgO-Al2O3-SiO2 2MgO·2Al2O3·5SiO2、4MgO·5Al2O3·2SiO2、3MgO·Al2O3·3SiO2 CaO-Al2O3-Ce2O3 2CaO·Al2O3·Ce2O3、2CaO·3Al2O3·Ce2O3 CaO-Al2O3-MgO 3CaO·MgO·2Al2O3、CaO·MgO·7Al2O3、CaO·2MgO·8Al2O3 CaO-Al2O3-SiO2 CaO·3SiO2·2Ce2O3 表 2 CaO·3SiO2·2Ce2O3化合物的热力学数据
Table 2. Thermodynamic data of CaO·3SiO2·2Ce2O3 compounds
化合物 H298/ (kJ·mol−1) S298 /[J.(mol·K)−1] Cp(T)=a+b×10−3T+c×105T−2 [J·(mol·K)−1] a b c CaO·3SiO2·2Ce2O3 −7330.568 466.137 477.948 67.814 −88.2 -
[1] Yue L, Wang L, Han J. Effects of rare earth on inclusions and corrosion resistance of 10 PCuRE weathering steel[J]. Journal of Rare Earths, 2010,(6):5. [2] Xu Y W, Song S H, Wang J W. Effect of rare earth cerium on the creep properties of modified 9Cr–1Mo heat-resistant steel[J]. Materials Letters, 2015,161(15):616−619. [3] Hamidzadeh M A, Meratian M, Saatchi A. Effect of cerium and lanthanum on the microstructure and mechanical properties of AISI D2 tool steel[J]. Materials Science and Engineering:A, 2013,571(1):193−198. [4] Qi J, Liu C J, Jiang M F. Viscosity-structure-crystallization of the Ce2O3-bearing calcium-aluminate-based melts with different contents of B2O3[J]. ISIJ International, 2018,58(1):186−193. doi: 10.2355/isijinternational.ISIJINT-2017-252 [5] Mitsutaka H, Kimihisa I. Thermodynamic data for steelmaking[M]. Japan: Tohoku University Press, 2010. [6] Kitano R, Ishii M, Motohiro U O, et al. Thermodynamic properties of the CaO-AlO1.5-CeO1.5 system[J]. ISIJ International, 2016,56(11):1893−1901. doi: 10.2355/isijinternational.ISIJINT-2016-201 [7] 田彦文, 翟秀静, 刘奎仁. 冶金物理化学 [M]. 北京: 化学工业出版社, 2007.Tian Yanwen, Zhai Xiujing, Liu Kuiren. Physical chemistry of metallurgy[M]. Beijing: Metallurgical Industry Press, 2007. [8] 魏庆成. 冶金热力学[M]. 重庆: 重庆大学出版社, 1996.Wei Qingcheng. Metallurgical thermodynamics [M]. Chongqing: Chongqing University Press, 1996. [9] 张鉴, 成国光, 王力军, 等. 冶金熔体的计算热力学 [M]. 北京: 冶金工业出版社, 1998.Zhang Jian, Cheng Guoguang, Wang Lijun, et al. Computational thermodynamics of metallurgical melts[M]. Beijing: Metallurgical Industry Press, 1998. [10] Yang X M, Duan J P, Shi C B, et al. A Thermodynamic model of phosphorus distribution ratio between CaO-SiO2-MgO-FeO-Fe2O3-MnO-Al2O3-P2O5 slags and molten steel during a top–bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory[J]. Metallurgical & Materials Transactions B, 2011,42(4):738−770. [11] Ma Deng, Wu Wei, Dai Shifan, et al. Activity-calculating model of vanadium slag system and its application[J]. Journal of Materials and Metallurgy, 2017,16(4):7. (马登, 吴巍, 戴诗凡, 等. 含钒渣系活度计算模型及应用[J]. 材料与冶金学报, 2017,16(4):7. doi: 10.14186/j.cnki.1671-6620.2017.04.001Ma Deng, Wu Wei, Dai Shifan, et al. Activity-calculating model of vanadium slag system and its application[J]. Journal of Materials and Metallurgy, 2017, 16(4): 7. doi: 10.14186/j.cnki.1671-6620.2017.04.001 [12] M Susa. Slag Atlas 2nd ed[M]. German: Verlag Stahleisen GmbH, 1995. [13] Lan X, Gao J, Du Y, et al. Thermodynamics and crystallization kinetics of REEs in CaO–SiO2–Ce2O3 system[J]. Journal of the American Ceramic Society, 2020,4:103. [14] Bale C W, Bélisle E, Chartrand P, et al. Reprint of: FactSage thermochemical software and databases, 2010–2016[J]. Calphad, 2016,55:1−19. doi: 10.1016/j.calphad.2016.07.004 [15] Li X, Yang L, Zhou Q, et al. A split-combination method for estimating the thermodynamic properties (Go and Ho) of multicomponent minerals[J]. Applied Clay Science, 2020,185:105406. doi: 10.1016/j.clay.2019.105406 [16] Leitner J, Chuchvalec P, Sedmidubsky D, et al. Estimation of heat capacities of solid mixed oxides[J]. Thermochimica Acta, 2002,395(1-2):27−46. doi: 10.1016/S0040-6031(02)00177-6 [17] Kubaschewski O, Uenal H. An empirical estimation of the heat capacities of inorganic compounds[J]. High Temperatures-High Pressures, 1977,9(3):361−365. [18] Zheng X, Liu C J. Thermodynamic properties assessment of CaO-Al2O3-Ce2O3 system[J]. Metallurgical and Materials Transactions B, 2021,52:3183−3192. doi: 10.1007/s11663-021-02245-z [19] Du Yong, Peng Jiaqing, Ji Jianying. Practice on 100 t ladle furnace[J]. China Metallurgy, 2006,(8):17−19. (杜勇, 彭家清, 姬健营. 100 t转炉LF精炼工艺的生产实践[J]. 中国冶金, 2006,(8):17−19. doi: 10.3969/j.issn.1006-9356.2006.08.005Du Yong, Peng Jiaqing, Ji Jianying. Practice on 100 t ladle furnace[J]. China Metallurgy, 2006(8): 17-19. doi: 10.3969/j.issn.1006-9356.2006.08.005 -





 下载:
下载: