Experimental study on precipitation of ammonium metavanadate in NaVO3-H2O system by ammonium bicarbonate and ammonium carbonate
-
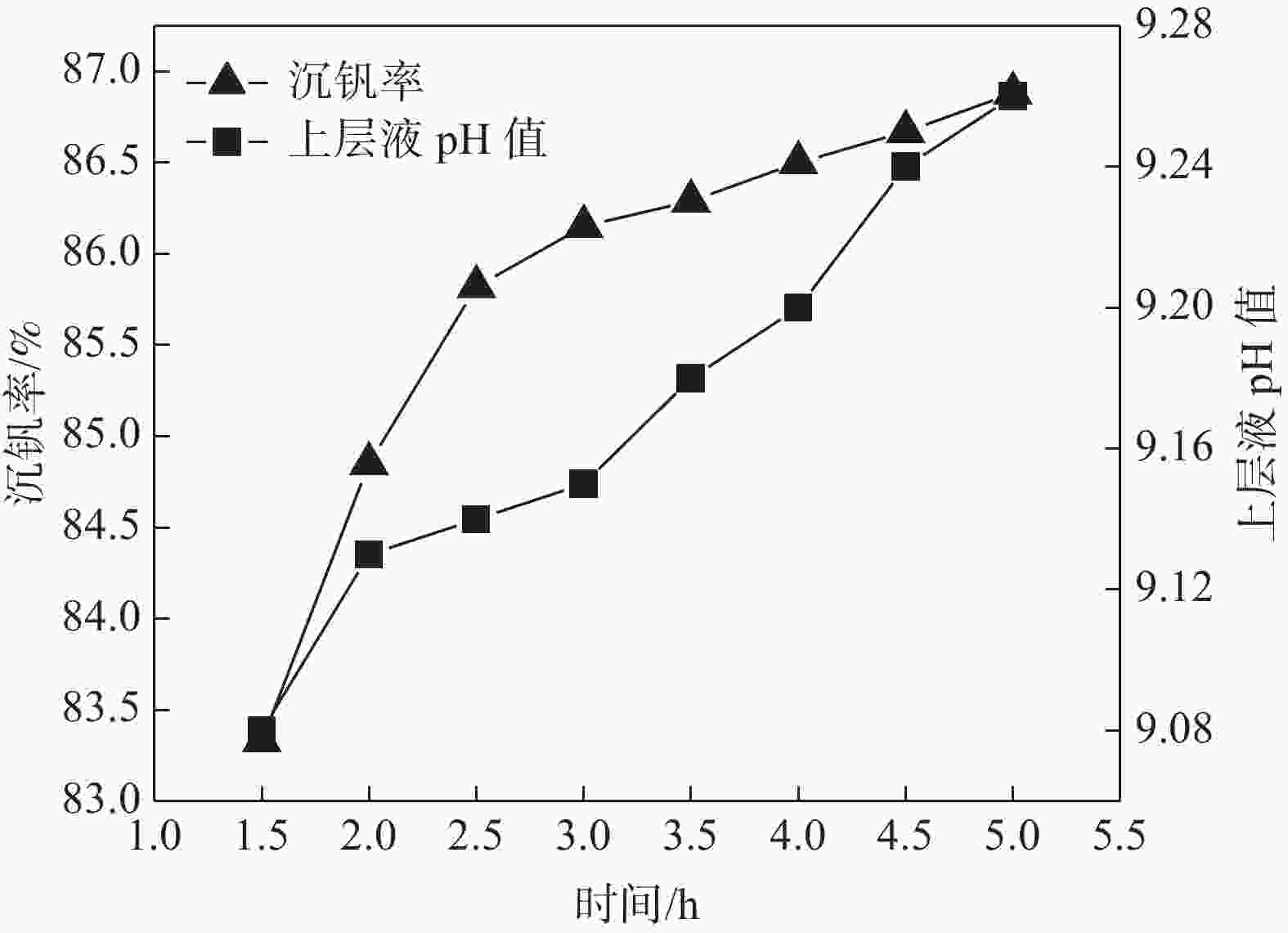
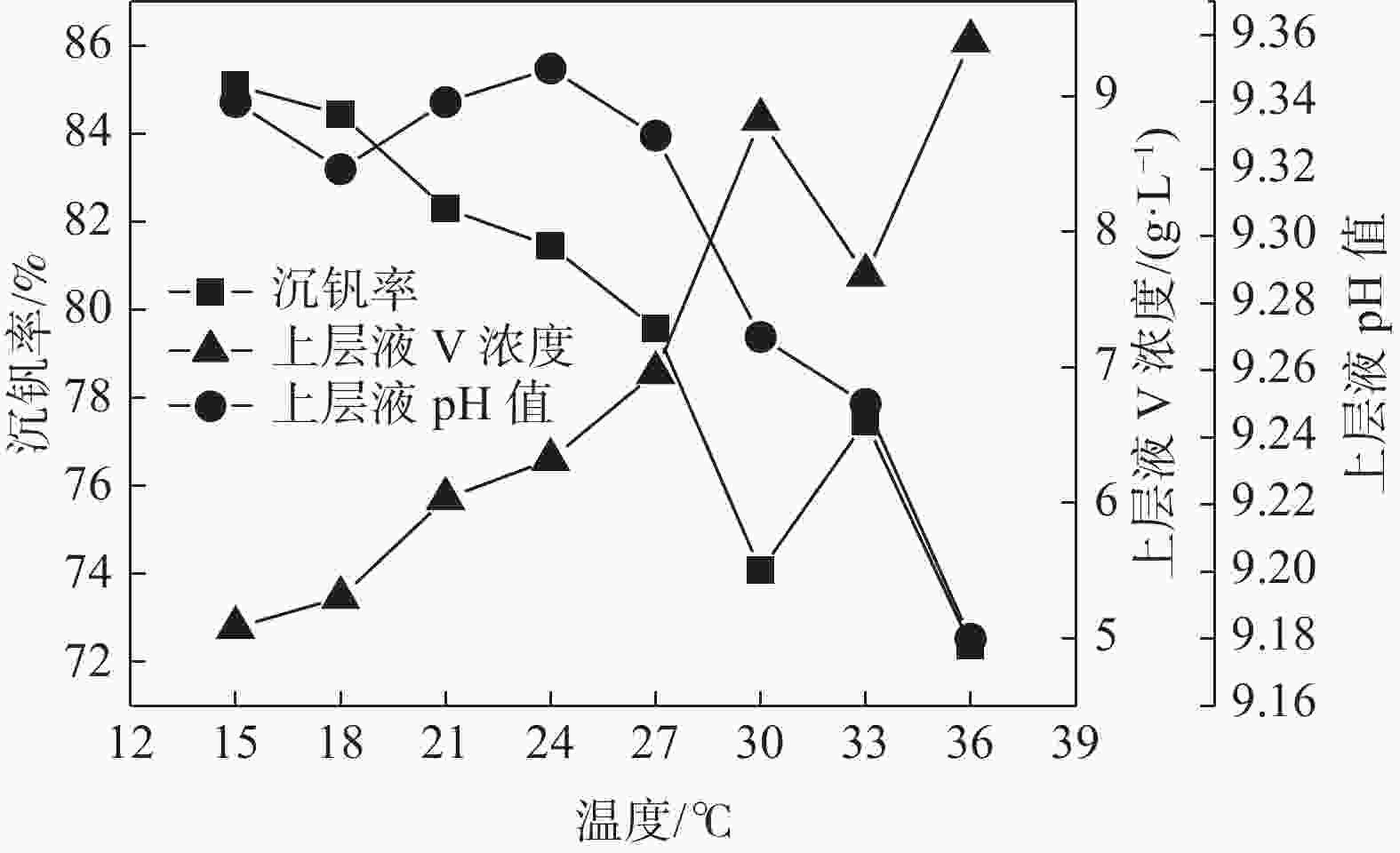
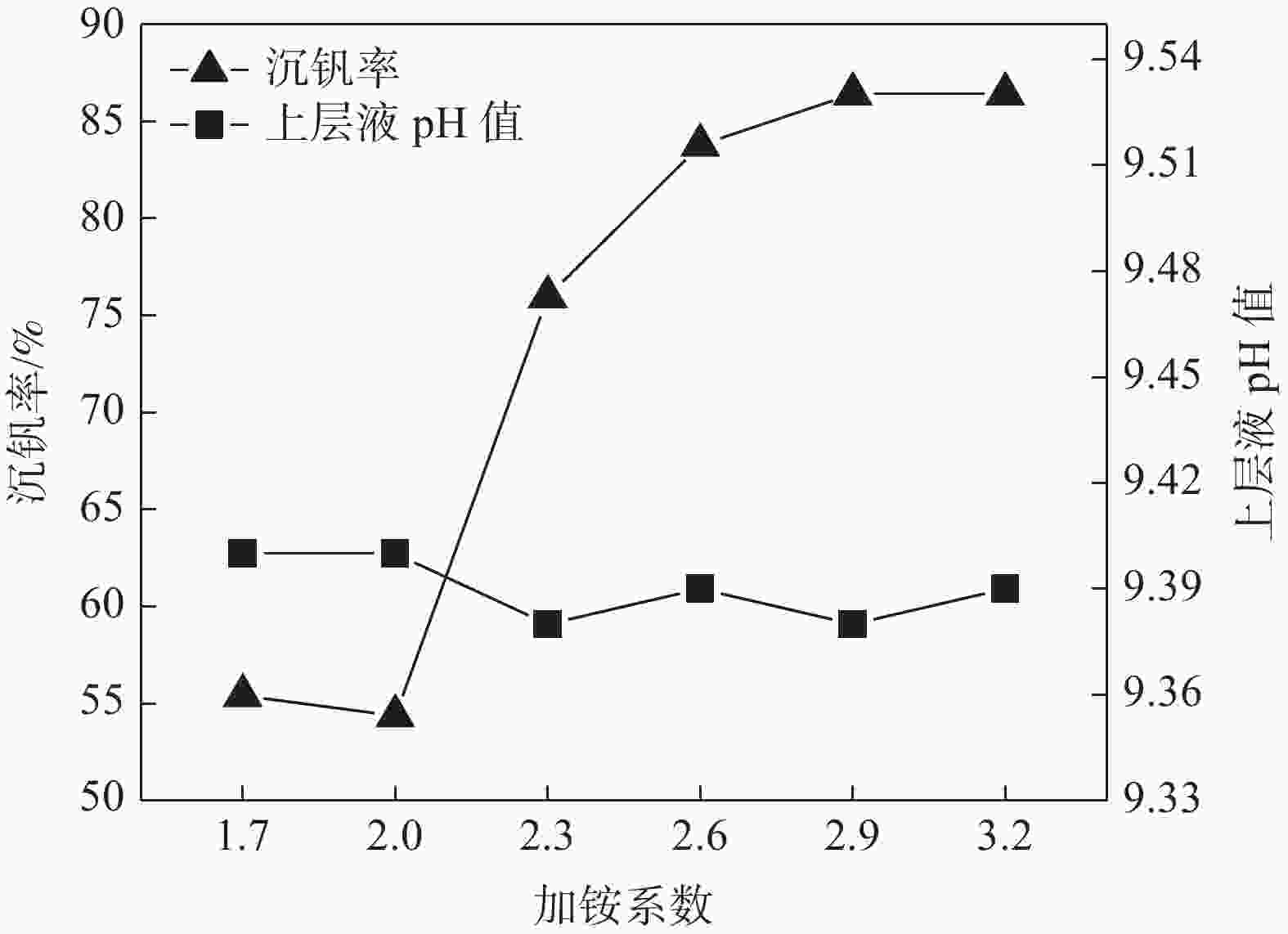
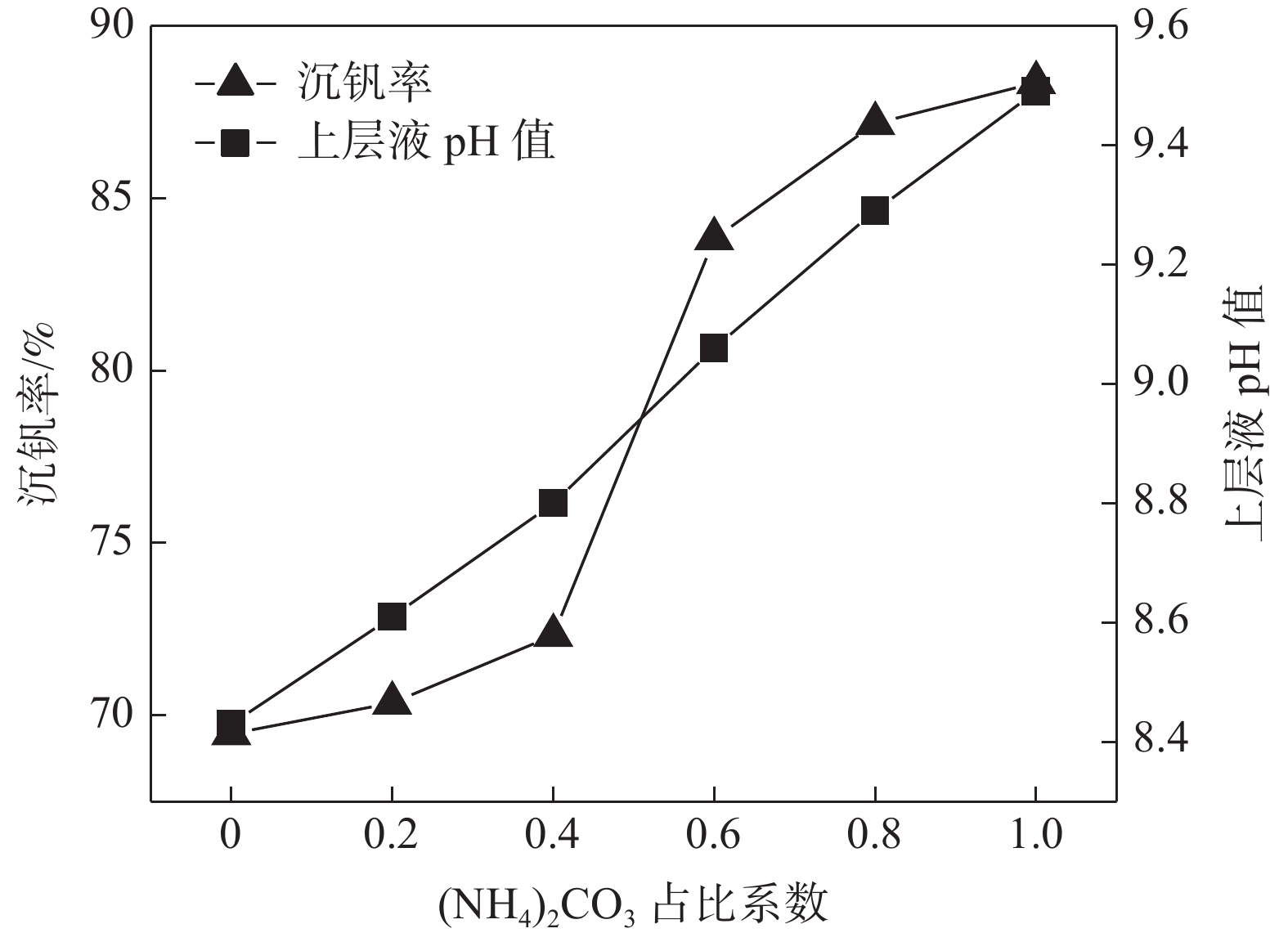
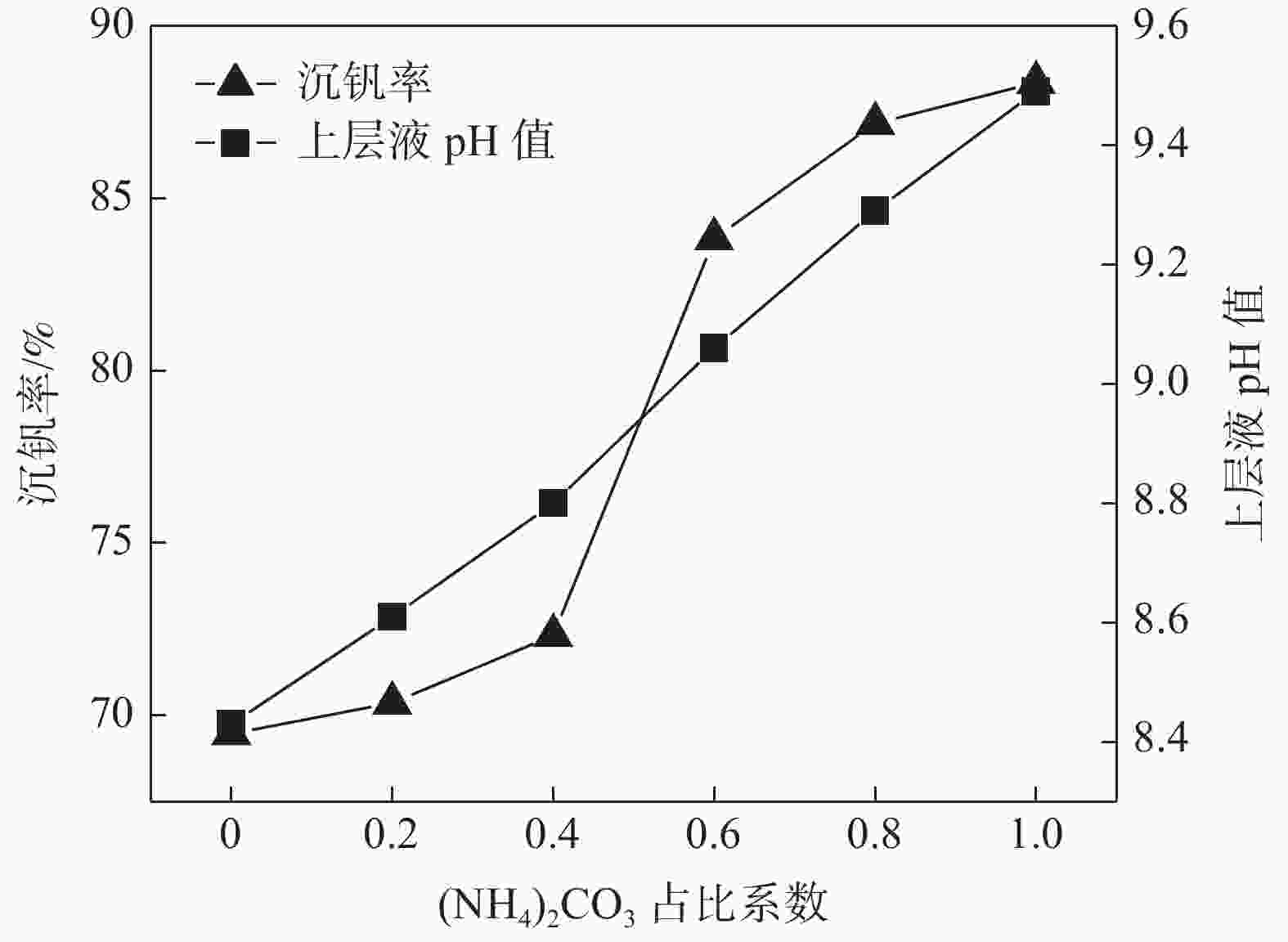
摘要: 针对钒渣钠法提钒得到的NaVO3-H2O溶液体系,利用碳酸氢铵/碳酸铵作为铵盐,考察了沉淀pH值、沉淀温度、结晶时间、加铵系数对偏钒酸铵结晶效果的影响。结果表明:在一定范围内提高溶液的pH值(9.1~9.5),可以改善偏钒酸铵的结晶率;在温度15~27 ℃、结晶时间>3 h、加铵系数不小于2.9条件下,可实现偏钒酸铵结晶率>85%,经煅烧获得的五氧化二钒纯度>99.3%,满足YB/T 5304—2017质量标准要求。Abstract: For the NaVO3-H2O solution system obtained by extracting vanadium from vanadium slag by sodium method, the effects of precipitation pH value, precipitation temperature, crystallization time, ammonium addition coefficient on the crystallization of ammonium metavanadate were investigated using ammonium bicarbonate and ammonium carbonate as ammonium salt. The results show that the crystallization rate of ammonium metavanadate can be improved by increasing the pH value of the solution (9.1–9.5) in a certain range. Under the conditions of temperature 15–27 ℃, crystallization time >3 h and ammonium addition coefficient no less than 2.9, the crystallization rate of ammonium metavanadate is higher than 85%, and the purity of vanadium pentoxide obtained by calcination ammonium metavanadate is higher than 99.3%, meeting the requirements of YB/T 5304—2017 quality standard.
-
表 1 沉钒原液主要成分
Table 1. Main components of vanadium precipitation solution
g/L V P Si Na 34.07 0.067 0.019 44.40 表 2 主要试验设备
Table 2. Main test equipment
设备 型号 厂家 智能数显恒温水浴锅 HH-5L 郑州长城科工贸有限公司 循环水式真空泵 SBH-3 郑州科泰实验设备有限公司 电动搅拌器 H2010-G 上海梅颖浦仪器仪表制造有限公司 贝意克马弗炉 MF-1100C 安徽贝意克设备技术有限公司 精密电子秤 MW-2002C 上海沪士电子有限公司 实验室pH计 PHSJ-3F 上海仪电科学仪器股份有限公司 表 3 pH沉钒试验偏钒酸铵主要成分
Table 3. Main components of ammonium metavanadate in pH vanadium precipitation test
样品名称 碳酸铵占比 w/% P NH4VO3 Si Na S 偏钒酸铵 0.0 <0.01 98.50 0.017 0.038 0.008 偏钒酸铵 0.2 <0.01 98.92 0.012 0.034 0.007 偏钒酸铵 0.4 <0.01 98.58 0.015 0.025 0.007 偏钒酸铵 0.6 <0.01 99.29 0.012 0.032 0.006 偏钒酸铵 0.8 <0.01 99.29 0.014 0.029 0.008 偏钒酸铵 1.0 <0.01 98.92 0.013 0.035 0.006 表 4 沉钒时间试验五氧化二钒主要成分
Table 4. Main components of vanadium pentoxide in vanadium precipitation time test
样品名称 沉钒时间 /h P /% Si /% Na /% S /% V2O5 /% 五氧化二钒 1.5 <0.01 0.031 0.048 0.011 99.42 五氧化二钒 2.0 <0.01 0.030 0.044 0.012 99.30 五氧化二钒 2.5 <0.01 0.033 0.043 0.014 99.53 五氧化二钒 3.0 <0.01 0.033 0.054 0.010 99.30 五氧化二钒 3.5 <0.01 0.031 0.062 0.011 99.53 五氧化二钒 4.0 <0.01 0.032 0.060 0.011 99.30 五氧化二钒 4.5 <0.01 0.030 0.046 0.013 99.44 五氧化二钒 5.0 <0.01 0.031 0.072 0.012 99.30 表 5 沉钒温度试验五氧化二钒主要成分
Table 5. Main components of vanadium pentoxide in temperature precipitation test
样品名称 沉钒温度 /℃ P /% Si /% Na /% S /% V2O5 /% 五氧化二钒 15 <0.01 0.033 0.064 0.014 99.42 五氧化二钒 18 <0.01 0.030 0.050 0.011 99.30 五氧化二钒 21 <0.01 0.033 0.039 0.010 99.76 五氧化二钒 24 <0.01 0.031 0.052 0.010 99.76 五氧化二钒 27 <0.01 0.034 0.044 0.015 99.64 五氧化二钒 30 <0.01 0.032 0.034 0.014 99.76 五氧化二钒 33 <0.01 0.031 0.029 0.012 99.53 五氧化二钒 36 <0.01 0.035 0.028 0.010 99.53 表 6 沉钒加铵系数试验五氧化二钒主要成分
Table 6. Main components of vanadium pentoxide in vanadium precipitation test with different ammonium addition
样品名称 加铵系数 P /% Si /% Na /% S /% V2O5 /% 五氧化二钒 1.7 <0.01 0.032 0.050 0.013 99.42 五氧化二钒 2.0 <0.01 0.034 0.052 0.016 99.64 五氧化二钒 2.3 <0.01 0.033 0.045 0.010 99.30 五氧化二钒 2.6 <0.01 0.035 0.042 0.012 99.76 五氧化二钒 2.9 <0.01 0.032 0.053 0.013 99.53 五氧化二钒 3.2 <0.01 0.035 0.046 0.011 99.53 -
[1] Sun Zhaohui. The view on the development of vanadium industry[J]. Panzhihua Science and Information, 2009,34(2):24−29. (孙朝晖. 钒产业发展之我见[J]. 攀枝花科技与信息, 2009,34(2):24−29. [2] Li Jing, Shi Zhe, Liang Chen, et al. Low calcification roasting sodium carbonate leaching vanadium extraction test[J]. Mining and Metallurgy, 2015,24(5):52−56. (李京, 施哲, 梁晨, 等. 低钙化焙烧—碳酸钠浸出提钒试验[J]. 矿冶, 2015,24(5):52−56. [3] 段冉. 高纯五氧化二钒的制备及偏钒酸铵结晶机理的研究[D]. 长沙: 中南大学, 2011.Duan Ran. Preparation of high purity vanadium pentoxide and study on crystallization mechanism of ammonium metavanadate[D]. Changsha: Central South University, 2011. [4] Yin Zhaoqian, Guo Jike, Chen Xiangquan, et al. Study on hydrolysis and precipitation of vanadium from sodium vanadate solution[J]. Iron Steel Vanadium Titanium, 2015,36(3):16−20. (殷兆迁, 郭继科, 陈相全, 等. 钠化钒液水解沉钒的研究[J]. 钢铁钒钛, 2015,36(3):16−20. [5] Wang Jun, Sun Zhaohui, Su Yi, et al. Study on roasting and leaching of vanadium slag with high calcium and phosphorus[J]. Rare Metals, 2015,39(11):1038−1042. (王俊, 孙朝晖, 苏毅, 等. 高钙高磷钒渣焙烧浸出的研究[J]. 稀有金属, 2015,39(11):1038−1042. [6] Wang Jun, Sun Zhaohui, Liu Jiayuan, et al. Experimental study on the recycling of vanadium precipitation wastewater from ammonium carbonate[J]. Iron Steel Vanadium Titanium, 2015,36(3):45−48, 61. (王俊, 孙朝晖, 刘佳媛, 等. 碳酸铵沉钒废水循环利用的试验研究[J]. 钢铁钒钛, 2015,36(3):45−48, 61. [7] Fu Zibi, Zheng Shili, Sun Zhaohui. Experimental study on preparation of V2O5 by leaching calcified clinker with sodium bicarbonate[J]. Iron Steel Vanadium Titanium, 2015,36(1):1−6. (付自碧, 郑诗礼, 孙朝晖. 碳酸氢钠浸出钙化熟料制备V2O5的试验研究[J]. 钢铁钒钛, 2015,36(1):1−6. [8] Guo Xuemei, Wang Shaona, Du Hao, et al. Cooling crystallization of ammonium metavanadate in ammonium bicarbonate solution[J]. Progress in Chemical Industry, 2018,37(3):853−860. (郭雪梅, 王少娜, 杜浩, 等. 碳酸氢铵溶液中偏钒酸铵的冷却结晶[J]. 化工进展, 2018,37(3):853−860. [9] 廖世明, 柏谈论. 国外钒冶金[M]. 北京: 冶金工业出版社, 1985: 32.Liao Shiming, Bai Tanlun. Vanadium metallurgy abroad[M]. Beijing: Metallurgical Industry Press, 1985: 32. [10] Zhao Chu, Feng Man, Wang Shaona, et al. Determination of NH4VO3 solubility in ternary system NH4HCO3-NH4VO3-H2O at 40 ℃ and 75 ℃[J]. Progress in Chemical Industry, 2014,33(6):1408−1412. (赵楚, 冯曼, 王少娜, 等. 40 ℃和75 ℃下三元体系NH4HCO3-NH4VO3-H2O中NH4VO3溶解度的测定[J]. 化工进展, 2014,33(6):1408−1412. [11] 郭雪梅. 偏钒酸铵冷却结晶分离的应用基础研究[D]. 天津: 天津大学, 2019.Guo Xuemei. Applied basic research on cooling crystallization separation of ammonium metavanadate[D]. Tianjin: Tianjin University, 2019. [12] Fu Zibi. Discussion on clean vanadium extraction process by blank roasting of vanadium slag[J]. Iron Steel Vanadium Titanium, 2019,40(4):17−23. (付自碧. 钒渣空白焙烧清洁提钒工艺探讨[J]. 钢铁钒钛, 2019,40(4):17−23. [13] Wang Shaona, Du Hao, Zheng Shili, et al. A new clean process for the preparation of vanadium oxide by sodium vanadate calcification ammonium carbide precipitation[J]. Journal of Chemical Engineering, 2017,(7):2781−2789. (王少娜, 杜浩, 郑诗礼, 等. 钒酸钠钙化-碳化铵沉法清洁制备钒氧化物新工艺[J]. 化工学报, 2017,(7):2781−2789. [14] Guo Jike, Yin Zhaoqian, Jiang Lin, et al. Study on separation technology of impurities in acid ammonium salt vanadium precipitation products[J]. Iron Steel Vanadium Titanium, 2018,39(5):16−23. (郭继科, 殷兆迁, 蒋霖, 等. 酸性铵盐沉钒产品中杂质分离技术研究[J]. 钢铁钒钛, 2018,39(5):16−23. -




 下载:
下载: