Effect of structural evolution of metatitanic acid on sulfur content during hydrolysis of industrial TiOSO4 solution
-
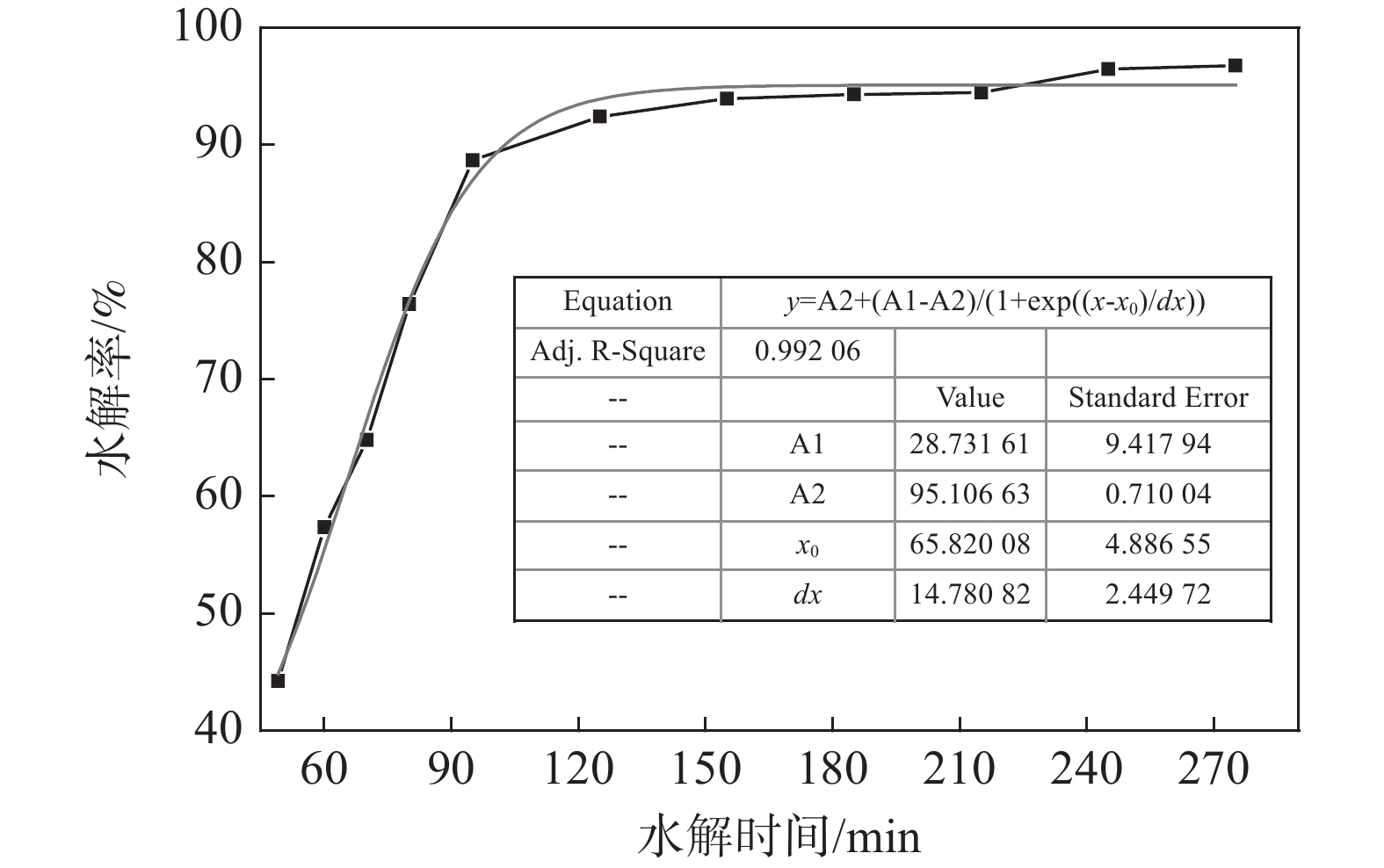
摘要: 采用外加晶种热水解工艺从工业钛液制得偏钛酸,研究了偏钛酸结构演变过程对硫含量的影响。水解过程分为快速水解段和慢速成熟阶段,水解率变化满足Boltzmann模型。随着水解进行,偏钛酸结构变化明显,颗粒聚集与调整使其结构更紧凑,胶体性质减弱,TiO2含量增大。偏钛酸颗粒的微孔与介孔结构由其一次聚集体聚集、堆积形成,孔道由硫酸盐与水等填充。硫酸根通过溶解于吸附水、吸附、键合等形式存在于偏钛酸中,硫含量随TiO2含量增大而逐渐减小,二者呈线性关系。Abstract: The effect of structural evolution of metatitanic acid on sulfur content was investigated by hydrolyzing the industrial TiOSO4 solution via thermal hydrolysis method with extra-adding seed. The hydrolysis process was divided into fast hydrolysis stage and slow maturation stage, the change of hydrolysis degree conformed to Boltzmann model. The structure of metatitanic acid changed obviously as the hydrolysis proceeded, its structure became more compact due to the aggregation and adjustment of the precipitated particles, with weaker colloidal property and higher TiO2 content. The microporous and mesoporous structures of metatitanic acid were formed by the aggregation and accumulation of its primary aggregates, and the pores were filled with sulfate and water. Sulfate radical existed in metatitanic acid was mainly in the form of dissolution in adsorbed water, adsorption, bonding, etc. The sulfur content decreased gradually with the increase of TiO2 content, showing a linear relationship.
-
Key words:
- metatitanic acid /
- sulfur content /
- industrial titanyl sulfate /
- hydrolysis /
- structural evolution
-
表 1 不同水解时间对水解率及偏钛酸结构与硫含量的影响
Table 1. Effect of hydrolysis time on hydrolysis degree, structure and S content of metatitanic acid
编号 水解时间/min 水解率/ % TiO2含量/ % L(101),MA /nm C轴的晶格应力/% DAV,MA /µm SBET /(m2.g−1) 平均孔径 /nm S含量/% 1# 49 44.23 49.92 9.3 1.666 1.49 259.3 3.394 12.07 2# 60 57.36 61.11 9.7 1.664 1.53 264.8 3.399 8.83 3# 70 64.82 65.72 10.1 1.662 1.60 267.9 3.408 7.88 4# 80 76.41 71.23 11.0 1.506 1.52 256.3 3.407 6.34 5# 95 88.69 72.04 11.6 1.445 1.53 277.7 3.401 6.13 6# 125 92.43 74.56 12.2 1.443 1.57 302.1 3.396 5.35 7# 155 93.94 78.45 12.3 1.428 1.62 300.0 3.400 3.61 8# 185 94.32 79.12 13.1 1.324 1.70 288.9 3.401 3.49 9# 215 94.45 80.01 13.9 1.328 1.43 298.6 3.351 3.41 10# 245 96.46 77.67 14.4 1.346 1.31 286.6 3.392 4.29 11# 275 96.78 79.04 15.3 1.285 1.26 276.0 3.405 3.78 -
[1] Cui W, Wang Y, He Z J, et al. A sol-gel route to prepare CeOx dot-decorated TiO2 pigment with improved weatherability[J]. Mater. Today Commun., 2022,31:103752. doi: 10.1016/j.mtcomm.2022.103752 [2] Bai Y, Mora-Sero I, Angelis F De, et al. Titanium dioxide nanomaterials for photovoltaic applications[J]. Chem. Rev., 2014,114:10095−10130. doi: 10.1021/cr400606n [3] Chen X, Mao S S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications[J]. Chem. Rev., 2007,107:2891−2959. doi: 10.1021/cr0500535 [4] Santacesatia E, Tonello M, Storti G, et al. Kinetics of titanium dioxide precipitation by thermal hydrolysis[J]. J. Colloid Interf. Sci., 1986,111:44−53. doi: 10.1016/0021-9797(86)90005-6 [5] Sathyamoorthy S, Moggridge G D, Hounslow M J. Particle formation during anatase precipitation of seeded titanyl sulfate solution[J]. Cryst. Growth Des., 2001,(1):123−129. doi: 10.1021/cg0000013 [6] Sathyamoorthy S, Moggridge G D, Hounslow M J. Controlling particle size during anatase precipitation[J]. AICHE J., 2001,47:2012−2024. doi: 10.1002/aic.690470912 [7] Tian C X. Internal influences of hydrolysis conditions on rutile TiO2 pigment production via short sulfate process[J]. Mater. Res. Bull., 2018,103:83−88. doi: 10.1016/j.materresbull.2018.03.025 [8] Sathyamoorthy S, Hounslow M J, Moggridge G D. Influence of stirrer speed on the precipitation of anatase particles from titanyl sulphate solution[J]. J. Cryst. Growth, 2001,223:225−234. doi: 10.1016/S0022-0248(01)00619-4 [9] Szilagyi I, Konigsberger E, May P M. Characterization of chemical speciation of titanyl sulfate solutions for production of titanium dioxide precipitates[J]. Inorg. Chem., 2009,48:2200−2204. doi: 10.1021/ic801722r [10] Grzmil B, Grela D, Kic B. Effects of processing parameters on hydrolysis of TiOSO4[J]. Pol. J. Chem. Technol., 2009,11(3):15−21. doi: 10.2478/v10026-009-0030-1 [11] Grzmil B, Grela D, Kic B. Formation of hydrated titanium dioxide from seeded titanyl sulphate solution[J]. Chem. Pap., 2009,63(2):217−225. doi: 10.2478/s11696-009-0009-7 [12] Hanaor D A H, Sorrell C C. Review of the anatase to rutile phase transformation[J]. J. Mater. Sci., 2011,46:855−874. doi: 10.1007/s10853-010-5113-0 [13] Grzmil B, Rabe M, Kic B, et al. Influence of phosphate, potassium, lithium, and aluminium on the anatase-rutile phase transition[J]. Ind. Eng. Chem. Res., 2007,46:1018−1024. doi: 10.1021/ie060188g [14] Gennari F C, Pasquevich D M. Enhancing effect of iron chlorides on the anatase-rutile transition in titanium dioxide[J]. J. Am. Ceram. Soc., 1999,82:1915−1921. doi: 10.1111/j.1151-2916.1999.tb02016.x [15] Chen K, Yan X H, Wu P S, et al. Effect of sulfate on crystal phase transition and crystal growth of titanium dioxide in metatitanic acid calcination[J]. Phase Transit., 2021,94(5):353−365. doi: 10.1080/01411594.2021.1934467 [16] Tian C X, Du J Q, Chen X H, et al. Influence of hydrolysis in sulfate process on titania pigment producing[J]. Trans. Nonferrous Met. Soc. China, 2009,(s3):829−833. doi: 10.1016/S1003-6326(10)60160-4 [17] Bavykin V D, Vera P, Alerxander V, et al. Effect of TiOSO4 hydrothermal hydrolysis conditions on TiO2 morphology and gas-phase oxidative activity[J]. Res. Chem. Intermediat., 2007,33:449−464. doi: 10.1163/156856707779238702 [18] Yang Xuan, Xue Tianyan, Wang Lina, et al. Preparation and hydrolysis of titanyl sulfate in novel process for production of titanium dioxide[J]. Wet Metallurgy, 2010,29(4):277−281. (杨轩, 薛天艳, 王丽娜, 等. 碱法钛白粉生产工艺中硫酸钛溶液的制备和水解[J]. 湿法冶金, 2010,29(4):277−281. doi: 10.13355/j.cnki.sfyj.2010.04.003 [19] Grzmil B U, Grela D, Kic B. Hydrolysis of titanium sulphate compounds[J]. Chem. Pap., 2008,62:18−25. doi: 10.2478/s11696-007-0074-8 [20] Zhu K R, Zhang M S, Chen Q, et al. Size and phonon confinement effects on low-frequency Raman mode of anatase TiO2 nanocrystal[J]. Phys. Lett. A, 2005,340:220−227. doi: 10.1016/j.physleta.2005.04.008 [21] Mandjoub N, Allen N, Kelly P, et al. SEM and Raman study of thermally treated TiO2 anatase nanopowders: Influence of calcination on photocatalytic activity[J]. J. of Photoch. Photobio. A, 2010,211:59−64. doi: 10.1016/j.jphotochem.2010.02.002 [22] Sivakumar S, Pillai P K, Mukundan P, et al. Sol-gel synthesis of nanosized anatase from titanyl sulfate[J]. Mater. Lett., 2002,57:330−335. doi: 10.1016/10.1016/S0167-577X(02)00786-3 [23] Yamaguchi T, Jin T, Ishida T, et al. Structural identification of acid sites of sulfur-promoted solid super acid and construction of its structure on silica support[J]. Mater. Chem. Phys., 1987,17:3−19. doi: 10.1016/0254-0584(87)90045-9 [24] Li X B, Nagaoka K, Lercher J A. Labile sulfates as key components in active sulfated zirconia for n-butane isomerization at low temperatures[J]. J. Catal., 2004,227:130−137. doi: 10.1016/j.jcat.2004.07.003 [25] Yang Y, Zhong H, Tian C X, et al. Single-step preparation and characterization of mesoporous Fe-doped sulfated titania[J]. Surf. Sci., 2011,605:1281−1286. doi: 10.1016/j.susc.2011.04.016 -





 下载:
下载: