Synthesis and sodium storage properties of V2O5/graphene nanocomposites
-
摘要: 五氧化二钒(V2O5)因其具有层状结构、高容量、低成本、资源丰富、高的Na+离子电导率、Na+嵌入/脱嵌时体积变化小、中等电位平台等优点而受到广泛关注。以V2O5和草酸为原料,采用水热法成功制备了V2O5/石墨烯纳米复合材料(V2O5/rGO)。结果表明:石墨烯与V2O5纳米线相互紧密交织,形成连续的V2O5-石墨烯网络结构;V2O5-石墨烯网络结构提供了快速的电子转移速度,扩展了电极材料与电解液的有效接触面积,提高了材料的导电性,缓冲了钠离子嵌入引起的体积变化,从而使V2O5/rGO纳米复合材料的储钠性能得到有效地改善(100 mA/g时,100次循环后,放电比容量为154 mAh/g)。Abstract: Vanadium pentoxide (V2O5) has attracted extensive attention due to its layered structure, abundant resources, high capacity, low cost, high Na+ ionic conductivity, small volume change during Na-ion insertion/extraction, and mesopotential platform. V2O5/graphene nanocomposites were successfully prepared by hydrothermal method, using oxalic acid and V2O5 as raw materials. The results show that the V2O5 nanowires and graphene are closely intertwined with each other, forming a V2O5-graphene nanocomposite with a tightly network structure. The structure can provide fast electron transfer kinetics, expand the effective contact area between the electrolyte and the electrode material, improve the conductivity of the material and buffer volume changes during Na-ion insertion/extraction. Thus, the electrochemistry properties of V2O5/rGO nanocomposites are effectively improved (Over 100 cycles, 154 mAh/g at 100 mA/g).
-
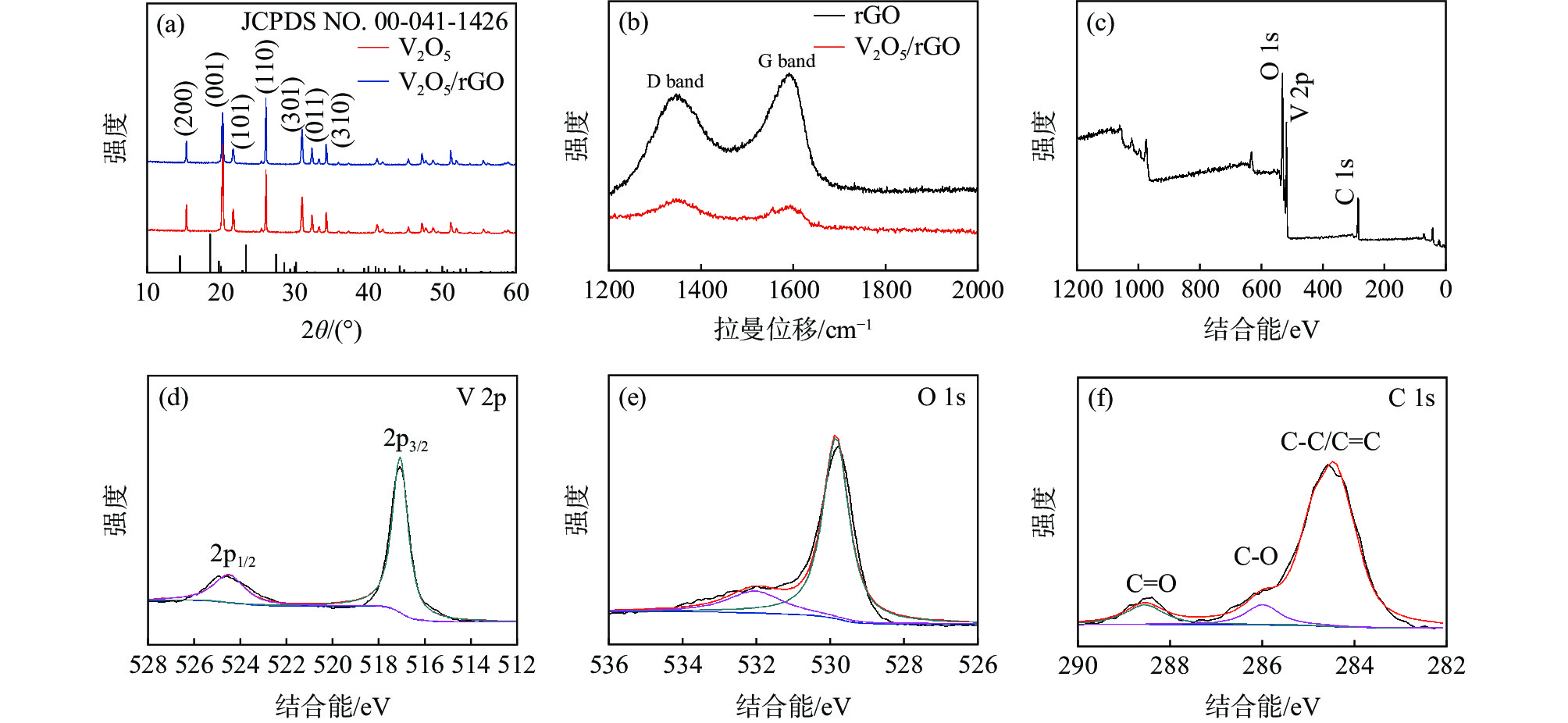
图 1 (a) V2O5和V2O5/rGO样品的XRD谱图,(b)石墨烯和V2O5/rGO样品的拉曼谱图,(c) V2O5/rGO纳米复合材料、(d) V 2p、(e) O 1s和 (f) C1s的XPS谱图
Figure 1. (a) XRD patterns of the V2O5 and V2O5/rGO samples, (b) Raman spectra of the graphene and V2O5/rGO samples, XPS scan spectra of the V2O5/rGO sample (c) full scan spectra, (d) V 2p, (e) O 1s and (f) C1s
图 3 (a) V2O5/rGO样品的循环伏安曲线,(b) V2O5样品的充放电曲线,(c)V2O5/rGO样品的充放电曲线,(d) V2O5和V2O5/rGO样品的循环性能,(e) V2O5和V2O5/rGO样品的倍率性能,(f) V2O5和V2O5/rGO样品的阻抗性能
Figure 3. (a) CV profiles of the V2O5 and V2O5/rGO samples, (b, c) Charge/discharge profiles of theV2O5 and V2O5/rGO samples, (d) Cycle performances of the V2O5 and V2O5/rGO samples, (e) Rate performance of the V2O5 and V2O5/rGO samples, (f) EIS of the V2O5 and V2O5/rGO samples
-
[1] Yabuuchi N, Kubota K, Dahbi M, et al. Research developments on sodium-ion batteries[J]. Chemical Reviews, 2014,114:11636−11682. doi: 10.1021/cr500192f [2] Zheng Hao, Chen Xiao, Yang Yun, et al. Self-assembled uniform double-shelled Co3V2O8 hollow nanospheres as anodes for high-performance Li-ion batteries[J]. Rare Metals, 2021,40:3485−3493. doi: 10.1007/s12598-021-01713-4 [3] Cocciantelli J M, Gravereau P, Doumerc J P, et al. Study on the preparation and characterization of a new polymorph of V2O5[J]. Journal of Solid State Electrochemistry, 1991,93:497−502. doi: 10.1016/0022-4596(91)90323-A [4] Hadjean R B, Renard M S, Emery N, et al. The richness of V2O5 polymorphs as superior cathode materials for sodium insertion[J]. Electrochimica Acta, 2018,270:129−137. doi: 10.1016/j.electacta.2018.03.062 [5] Zhu H L, Lee K T, Hitz G T, et al. Free-standing Na2/3Fe1/2Mn1/2O2@Graphene film for a sodium-ion battery cathode[J]. ACS Applied Materials Interfaces, 2014,6(6):4242−4247. doi: 10.1021/am405970s [6] Park Y U, Seo D H, Kwon H S, et al. A new high-energy cathode for a Na-ion battery with ultrahigh stability[J]. Journal of the American Chemical Society, 2013,135(37):13870−13878. doi: 10.1021/ja406016j [7] Su D, Zhao Y, Zhang R, et al. Dimension meditated optic and catalytic performance over vanadium pentoxides[J]. Applied Surface Science, 2016,389:112−117. doi: 10.1016/j.apsusc.2016.07.069 [8] Park B, Oh S M, Jo Y K, et al. Efficient electrode material of restacked Na–V2O5–graphene nanocomposite for Na-ion batteries[J]. Materials Letters, 2016,178(1):79−82. [9] Horrocks G A, Likely M F, Velazquez J M, et al. Finite size effects on the structural progression induced by lithiation of V2O5: a combined diffraction and Raman spectroscopy study[J]. Journal of Materials Chemistry A, 2013,1(48):15265−15277. doi: 10.1039/c3ta13690f [10] Raju V, Rains J, Gates C, et al. Superior cathode of sodium-ion batteries: Orthorhombic V2O5 nanoparticles generated in nanoporous carbonby ambient hydrolysis deposition[J]. Nano Letter, 2014,14(7):4119−4124. doi: 10.1021/nl501692p [11] Zhao Z J, Li D L, Wang C Z, et al. Hierarchical V2O5 microspheres: A pseudocapacitive cathode material for enhanced sodium ion storage[J]. Journal of Alloys and Compounds, 2022,895:162617. doi: 10.1016/j.jallcom.2021.162617 [12] Wei Q, Jiang Z, Tan S, et al. Lattice breathing inhibited layered vanadium oxide ultrathin nanobelts for enhanced sodium storage[J]. ACS Applied Materials Interfaces, 2015,7(33):18211−18217. doi: 10.1021/acsami.5b06154 [13] Zhang X L, Liu X X, Yang C, et al. A V2O5-nanosheets-coated hard carbon fiber fabric as high performance anode for sodium ion battery[J]. Surface and Coatings Technology, 2019,358(25):661−666. [14] Ali G, Lee J H, Oh S H, et al. Investigation of the Na intercalation mechanism into nano-sized V2O5/C composite cathode material for Na-ion batteries[J]. ACS Applied Materials Interfaces, 2016,8(9):6032−6039. doi: 10.1021/acsami.5b11954 [15] Wang L H, Wang Y L, Zhao Y J, et al. Freeze-drying method to synthesize V2O5/graphene composites toward enhanced sodium ion storage[J]. Ceramics International, 2018,44(18):23279−23283. doi: 10.1016/j.ceramint.2018.08.344 [16] Nagaraju D H, Wang Q, Beaujuge P, et al. Two-dimensional hetero structures of V2O5 and reduced graphene oxide as electrodes for high energy density asymmetric super capacitors[J]. Journal of Materials Chemistry A, 2014,2:17146−17152. doi: 10.1039/C4TA03731F [17] Jing Y, Zhou Z, Cabrera C R, et al. Graphene, inorganic graphene analogs and their composites for lithium ion batteries[J]. Journal of Materials Chemistry A, 2014,2:12104−12122. doi: 10.1039/C4TA01033G [18] Allen M J, Tung V C, Kaner R B. Honeycomb carbon: A review of garphene[J]. Chemical Reviews, 2010,110:132−145. doi: 10.1021/cr900070d [19] Zheng H, Chen X, Li L, et al. Synthesis of NiS2/reduced graphene oxide nanocomposites as anodes materials for high-performance sodium and potassium ion batteries[J]. Materials Research Bulletin, 2021,142:111430. doi: 10.1016/j.materresbull.2021.111430 [20] Ding C, Zhao Y, Yan D, et al. An insight into the convenience and efficiency of the freeze-drying route to construct 3D graphene-based hybrids for lithium-ion batteries[J]. Electrochimica Acta, 2016,221:124−132. doi: 10.1016/j.electacta.2016.10.054 -





 下载:
下载: