Non-isothermal reduction kinetics of carbon-coated vanadium-titanium magnetite powder
-
摘要: 为了准确探究钒钛磁铁矿粉碳还原反应的动力学,试验采用水热法,在不添加任何改性剂的情况下,以葡萄糖为碳源制备出了碳包覆钒钛磁铁矿粉,并利用高温综合热分析仪分别测量了5、7.5、10、12.5、15 K/min五种不同升温速率下的碳包覆钒钛磁铁矿粉的升温失重曲线。结合FWO公式和Coats-Redfern(CR)公式计算试验数据,进而计算碳包覆钒钛磁铁矿粉的动力学相关参数。结果表明:在一定温度区间内碳包覆钒钛磁铁矿粉的失重率与碳包覆量和升温速率成正比,此反应中的活化能约为73.533 kJ/mol,其反应机理是三级化学反应的模型。Abstract: To accurately explore the dynamics of carbon reduction reaction of vanadium-titanium magnetite powder, the test was conducted by hydrothermal method without adding any modifier. Carbon-coated vanadium-titanium magnetite powder was prepared with glucose as carbon source. The heating curves of weightlessness of carbon-coated vanadium-titanium magnetite powder obtained under different heating rate of 5, 7.5, 10, 15, 12.5, 1 5 K/min were measured by high temperature comprehensive thermal analyzer . Combined with the FWO formula and CR formula, the experimental data were calculated,as well as the kinetic parameters of carbon-coated vanadium-titanium magnetite powder. The results show that the weight loss rate of carbon-coated vanadium-titanium magnetite powder in a certain temperature range is proportional to the amount of carbon coating and the heating rate. The activation energy in this reaction is about 73.533 kJ/mol with a reaction mechanism of tertiary chemical reaction model.
-
Key words:
- vanadium-titanium magnetite powder /
- non-isothermal reduction /
- carbon coating /
- dynamics
-
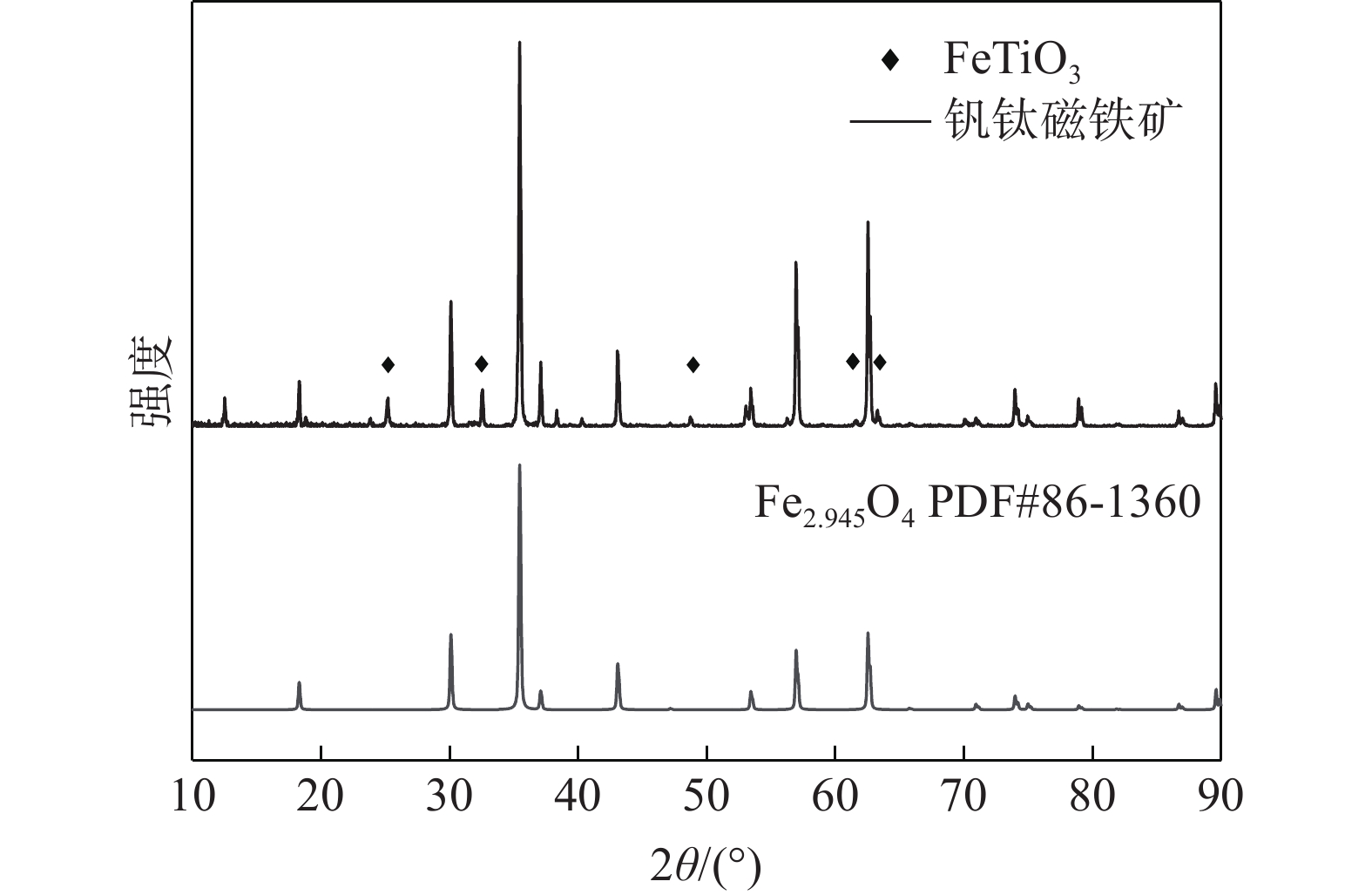
表 1 钒钛磁铁矿主要化学成分
Table 1. Main components of vanadium-titanium magnetite
% TFe TiO2 SiO2 Al2O3 V2O5 H2O P 62.03 6.88 1.71 2.15 0.75 9.35 0.02 表 2 常见固-固相反应模型
Table 2. Common solid-solid reaction models
Symbol Mechanism fuction G(α) D1 一维扩散(片状颗粒) $ {{\alpha}}^{{2}} $ D2 二维扩散(柱状颗粒) $ \left[{(1-\alpha)}{\ln}{(1-\alpha)}\right]{+\alpha} $ 1 D3 三维扩散Jander方程 $ {\left[{(1-}{\left({1- \alpha}\right)}^{\frac{{1}}{{3}}}\right]}^{{2}} $ 2 D3 三维扩散G-B方程 $ {1-}\dfrac{{2\alpha}}{{3}}{-}{\left({1- \alpha}\right)}^{\frac{{2}}{{3}}} $ A1 形核(n=1) $ {-}{\ln}{(1-\alpha)} $ A2/3 形核(n=3/2) $ {\left[{-}{\ln}{(1-\alpha)}\right]}^{\frac{{3}}{{2}}} $ A3/2 形核(n=2/3) $ {\left[{-}{\ln}{(1-\alpha)}\right]}^{\frac{2}{3}} $ A2 形核(n=1/2) $ {\left[{-}{\ln}{(1-\alpha)}\right]}^{\frac{1}{{2}}} $ A3 形核(n=1/3) $ {\left[{-}{\ln}{(1-\alpha)}\right]}^{\frac{1}{3}} $ R2 二维界面反应(柱状颗粒) $ {1-}{\left({1- \alpha}\right)}^{\frac{{1}}{2}} $ R3 三维界面反应(球状颗粒) $ {1-}{\left({1- \alpha}\right)}^{\frac{1}{{3}}} $ P2 幂律分布 $ {{\alpha}}^{\frac{{1}}{{2}}} $ P3 幂律分布 $ {{\alpha}}^{\frac{{1}}{{3}}} $ C2 化学反应 $ {{(1-\alpha)}}^{{-1}}{-1} $ C1.5 化学反应 $ {{(1-\alpha)}}^{{-}\frac{{1}}{{2}}} $ 表 3 碳包覆钒钛磁铁矿粉反应机理模型函数拟合结果(转化率为0.1~0.4)
Table 3. Model function fitting results of reaction mechanism of carbon-coated vanadium-titanium magnetite powder (Conversion rate 0f 0.1~0.4)
$ G(\propto ) $ $ {E}_{\beta \to 0}/ $
$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1} $)$ \beta =5$ ℃·mol−1 $ \beta =7.5$ ℃·mol−1 $ \beta =10$ ℃·mol−1 $ \beta =12.5$ ℃·mol−1 $ \beta =15$ ℃·mol−1 E/$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1}) $ $ R $ E/$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1}) $ $ R $ E/$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1}) $ $ R $ E/$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1}) $ $ R $ E/$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1}) $ $ R $ 1 44.820 44.927 0.983 47.524 0.981 47.952 0.974 47.493 0.980 47.857 0.983 2 46.489 46.581 0.984 49.272 0.982 49.750 0.976 49.238 0.982 49.597 0.984 3 24.128 24.335 0.997 25.721 0.996 25.775 0.995 25.777 0.996 26.065 0.996 4 47.096 47.183 0.985 49.907 0.983 50.404 0.977 49.873 0.982 50.229 0.984 5 31.984 32.141 0.995 33.979 0.993 34.209 0.991 34.010 0.993 34.307 0.993 6 41.084 41.191 0.991 43.559 0.988 43.971 0.985 43.556 0.988 43.876 0.989 7 25.917 26.107 0.997 27.593 0.996 27.701 0.995 27.647 0.996 27.928 0.996 8 22.883 23.090 0.998 24.399 0.997 24.448 0.997 24.465 0.997 24.738 0.997 9 19.850 20.073 0.998 21.206 0.998 21.194 0.998 21.283 0.998 21.549 0.997 10 30.595 30.767 0.994 32.528 0.993 32.714 0.990 32.560 0.992 32.863 0.993 11 31.048 31.214 0.994 33.001 0.993 33.201 0.990 33.033 0.993 33.334 0.993 12 21.542 21.761 0.998 22.996 0.998 23.003 0.997 23.063 0.998 23.341 0.998 13 18.956 19.187 0.998 20.270 0.998 20.230 0.998 20.348 0.998 20.617 0.998 14 35.036 35.163 0.995 37.171 0.993 37.499 0.992 37.199 0.993 37.482 0.993 15 16.650 16.879 0.986 17.819 0.987 17.776 0.988 17.916 0.987 18.154 0.986 表 4 碳包覆钒钛磁铁矿粉反应机理模型函数拟合结果(转化率为0.4~0.9)
Table 4. Model function fitting results of reaction mechanism of carbon-coated vanadium-titanium magnetite powder (Conversion rate of 0.4~0.9)
$ G(\propto ) $ $ {E}_{\beta \to 0}/ $
$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1} $)$ \beta =5$ ℃·mol−1 $ \beta =7.5$ ℃·mol−1 $ \beta =10$ ℃·mol−1 $ \beta =12.5$ ℃·mol−1 $ \beta =15$ ℃·mol−1 E/$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1}) $ $ R $ E/$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1}) $ $ R $ E/$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1}) $ $ R $ E/$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1}) $ $ R $ E/$ (\mathrm{k}\mathrm{J}\cdot{\mathrm{m}\mathrm{o}\mathrm{l}}^{-1}) $ $ R $ 1 82.284 90.530 0.979 103.377 0.984 94.844 0.986 104.644 0.989 113.852 0.993 2 96.625 106.940 0.966 122.795 0.972 112.311 0.976 124.452 0.981 135.938 0.986 3 41.976 44.997 0.988 49.790 0.991 46.858 0.992 50.448 0.994 53.628 0.996 4 75.247 114.547 0.958 131.812 0.966 120.434 0.970 133.676 0.976 146.231 0.982 5 80.735 89.333 0.935 102.245 0.943 94.046 0.951 103.954 0.957 113.274 0.963 6 110.191 122.884 0.925 141.870 0.935 129.637 0.943 144.255 0.951 158.153 0.958 7 61.097 66.965 0.947 75.829 0.952 70.319 0.959 77.086 0.964 83.354 0.968 8 51.278 55.782 0.955 62.621 0.960 58.455 0.965 63.652 0.969 68.394 0.973 9 41.459 44.598 0.967 49.412 0.969 46.592 0.974 50.218 0.976 53.435 0.979 10 64.403 70.539 0.965 79.955 0.970 73.949 0.975 81.125 0.979 87.789 0.983 11 59.383 76.263 0.956 86.740 0.962 80.064 0.968 88.068 0.972 95.539 0.977 12 36.937 39.305 0.991 43.091 0.992 40.860 0.993 43.674 0.995 46.100 0.996 13 31.899 33.614 0.993 36.393 0.994 34.861 0.995 36.899 0.996 38.572 0.997 14 125.832 141.408 0.866 164.095 0.880 149.888 0.893 167.446 0.903 184.203 0.912 15 53.487 64.744 0.869 64.744 0.875 68.382 0.890 75.075 0.894 81.275 0.899 -
[1] Gao Yongzhang. Vanadium resources and its supply and demand situation in China[J]. China Mining Magazine, 2019,28(S2):5−10. (高永璋. 中国钒矿资源及供需形势分析[J]. 中国矿业, 2019,28(S2):5−10. [2] Wang Xun, Han Yuexin, Li Yanjun, et al. Research status on comprehensive development and utilization of vanadium-titanium magnetite[J]. Metal Mine, 2019,(6):33−37. (王勋, 韩跃新, 李艳军, 等. 钒钛磁铁矿综合利用研究现状[J]. 金属矿山, 2019,(6):33−37. [3] Wang Shuai, Guo Yufeng, Jiang Tao, et al. Comprehensive utilization and industrial development direction of vanadium - titanium magnetite[J]. China Metallurgy, 2016,26(10):40−44. (王帅, 郭宇峰, 姜涛, 等. 钒钛磁铁矿综合利用现状及工业化发展方向[J]. 中国冶金, 2016,26(10):40−44. [4] Peng Yingjian, Lv Chao. Present situation and progress of comprehensive utilization of vanadium - titanium magnetite[J]. Mining Researchand Development, 2019,39(5):130−135. (彭英健, 吕超. 钒钛磁铁矿综合利用现状及进展[J]. 矿业研究与开发, 2019,39(5):130−135. [5] Zhang Xiaowei, Zhang Wanyi, Tong Ying, et al. Current situation and utilization trend of global titanium resources[J]. Consevation and Utilization of Mineral Resources, 2019,39(5):68−75. (张晓伟, 张万益, 童英, 等. 全球钛矿资源现状与利用趋势[J]. 矿产保护与利用, 2019,39(5):68−75. [6] Bai Ruiguo. Application and prospect of vanadium & titanium new material[J]. Hebei Metallurgy, 2019,(3):1−8. (白瑞国. 钒钛新材料的应用及展望[J]. 河北冶金, 2019,(3):1−8. [7] Zhu Junshi. Beneficiation and comprehensive utilization of vanadium-titanium magnetite[J]. Metal Mine, 2000,(1):1−5,11. (朱俊士. 钒钛磁铁矿选矿及综合利用[J]. 金属矿山, 2000,(1):1−5,11. doi: 10.3321/j.issn:1001-1250.2000.01.001 [8] Xu Guangkui. Development and research of vanadium titanium application in cast steel[J]. Iron Steel Vanadium Titanium, 1989,10(3):1−8. (许光奎. 钒钛在铸钢中应用的开发与研究[J]. 钢铁钒钛, 1989,10(3):1−8. doi: 10.7513/j.issn.1004-7638.1989.03.001 [9] Zhang Guangming, Feng Keqin, Deng Weilin, et al. Thermodynamic analysis on ferro-matrix composites by in-situ carbothermal reduction from vanadium-titanium magnetite[J]. Journalof Sichuan University(Engineering Science Edition), 2012,44(4):191−196. (张光明, 冯可芹, 邓伟林, 等. 钒钛磁铁矿碳热合成铁基复合材料的热力学分析[J]. 四川大学学报(工程科学版), 2012,44(4):191−196. [10] Zheng Fuqiang, Chen Feng, Guo Yufeng, et al. Kinetics of hydrochloric acid leaching of titanium from titanium-bearing electric furnace slag[J]. JOM, 2016,68(5):1476−1484. doi: 10.1007/s11837-015-1808-7 [11] 杨勇霞. 钒钛磁铁矿精矿直接还原技术的研究[D]. 沈阳: 东北大学, 2014.Yang Yongxia. Research on the direct reduction technologyof vanadium-titanium magnetite concentrate[D]. Shenyang: Northeastern University, 2014. [12] Sun Haoyan, Zhu Qingshan, Li Hongzhong. The technical state and development trend of the direct reduction of titanomagnetite by fluidized bed[J]. The Chinese Journal of Process Engineering, 2018,18(6):1146−1159. (孙昊延, 朱庆山, 李洪钟. 钒钛磁铁矿流态化直接还原技术现状与发展趋势[J]. 过程工程学报, 2018,18(6):1146−1159. [13] Hong Lukuo, Qi Yuanhong, Sun Caijiao, et al. Research on smelting-separation for metallized pellets of vanadium-titanium magnetite[J]. Iron Steel Vanadium Titanium, 2017,38(5):101−107. (洪陆阔, 齐渊洪, 孙彩娇, 等. 钒钛磁铁矿金属化球团还原熔分试验研究[J]. 钢铁钒钛, 2017,38(5):101−107. doi: 10.7513/j.issn.1004-7638.2017.05.019 [14] Wei R F, Cang D Q, Zhang L L, et al. Staged reaction kinetics and characteristics of iron oxide direct reduction by carbon[J]. International Journal of Minerals, Metallugryand Materials, 2015,22(10):1025−1032. [15] Liu Ran, Li Chao, Lv Qing, et al. Study on differential thermal analysis and single burning experiment of vanadium-titanium ore powder[J]. Iron Steel Vanadium Titanium, 2014,35(4):71−76. (刘然, 李超, 吕庆, 等. 承德钒钛磁铁矿粉基础特性及烧结试验研究[J]. 钢铁钒钛, 2014,35(4):71−76. doi: 10.7513/j.issn.1004-7638.2014.04.014 [16] Xu Xun. Research on direct reduction of vanadic titanomagnetite[J]. Iron Steel Vanadium Titanium, 2007,28(3):37−41. (薛逊. 钒钛磁铁矿直接还原实验研究[J]. 钢铁钒钛, 2007,28(3):37−41. doi: 10.3969/j.issn.1004-7638.2007.03.009 [17] Luo Shiyong, Zhang Jiayun, Zhou Tuping. Models for kinetic analyses of solid-solid reactions and their applications[J]. Materials Reports, 2000,14(4):6−7. (罗世永, 张家芸, 周土平. 固/固相反应动力学模型及其应用[J]. 材料导报, 2000,14(4):6−7. doi: 10.3321/j.issn:1005-023X.2000.04.004 [18] Han Y Q, Liu N A. New iso conversional method for evaluating unambiguous activation energy values for solid decomposition[J]. Fire Safety Science, 2011,20(1):9−15. [19] 李金莲. 铁氧化物/碳混合物还原的热分析质谱法研究及热分析动力学解析[D]. 鞍山: 辽宁科技大学, 2008.Li Jinlian. Study on the reduction of iron oxide/carbon composites by thermal analysis-mass spectrometry and analysis of thermal analysis kinetics[D]. Anshan: University of Science and Technology Liaoning, 2008. [20] 伦志刚. 钒钛铁精矿多层含碳球团转底炉内直接还原工艺研究[D]. 重庆: 重庆大学, 2013.Lun Zhigang. Research on direct reduction of multilayer carbon-containing pellets of V-Ti iron concentrate in rotary hearth furnace[D]. Chongqing : Chongqing University, 2013. -





 下载:
下载: