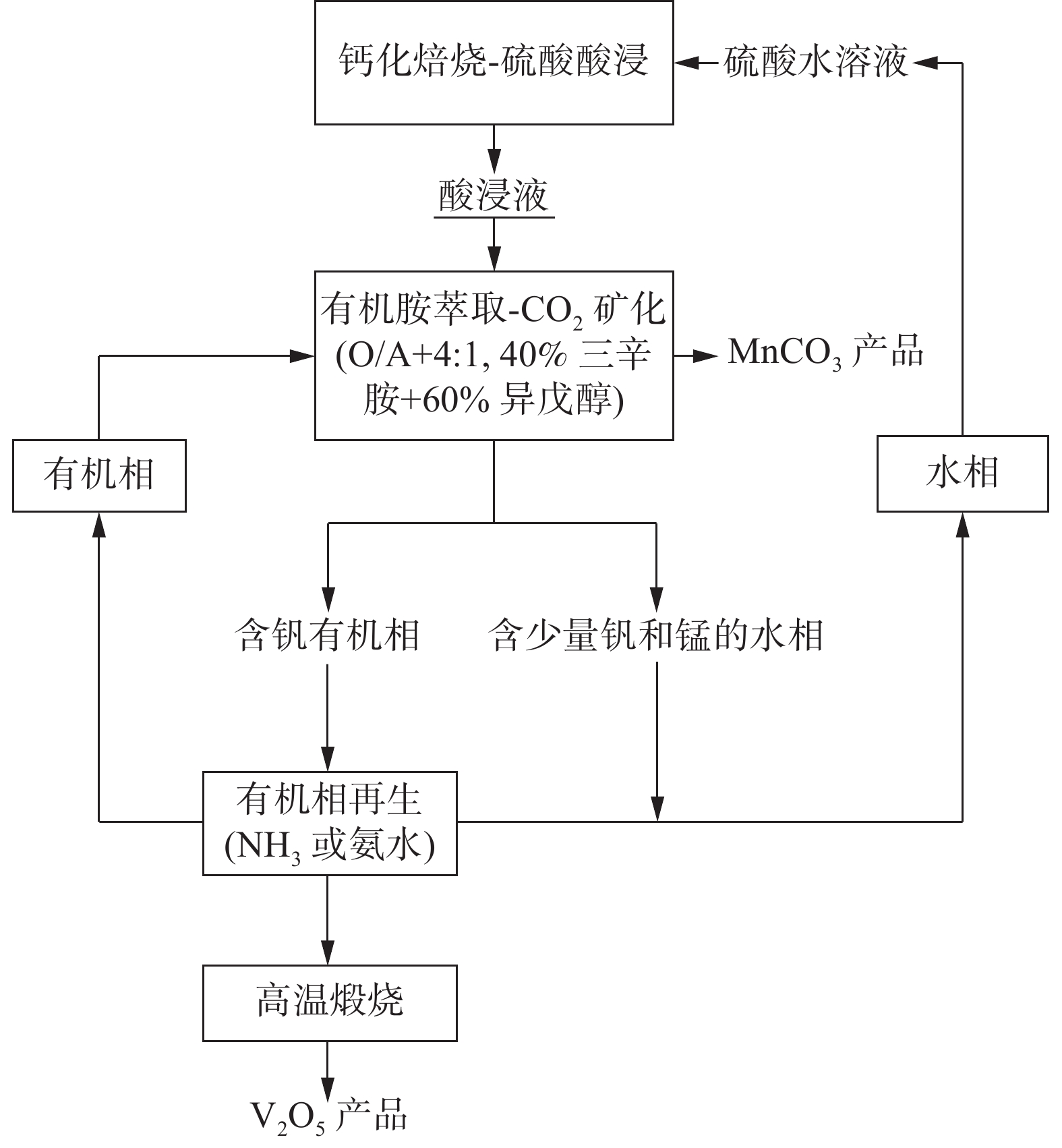
Separation of V and Mn in calcification roasting-acid leachate of V slag by simultaneous amine extraction and CO2 mineralization
-
摘要: 钒渣钙化焙烧酸浸液中钒锰浓度高,针对钒锰分离难度大、成本高的问题,提出并研究了胺萃取耦合CO2矿化分离钒锰新工艺。分析了钒锰分离过程的化学基础,研究了酸浸液中各金属元素在有机相、水相和固相中的分配规律,考察了钒锰浓度、胺再生等对钒锰分离效率的影响,并对该工艺的技术经济性进行了初步评估。 结果表明,对于高锰浓度酸浸液,钒的萃取率可达99%;胺再生循环使用5次后,钒的萃取率仍保持在97%,锰的沉淀率为96%,该工艺具有较高的技术性和经济性。Abstract: The efficient separation of vanadium and manganese from an acid leachate of the calcification roasting vanadium slag is quite difficult and costly, particularly for those leachates with high vanadium and manganese concentrations. A novel process was investigated for the separation of vanadium and manganese using trioctylamine and CO2. The chemical separation basic of V and Mn was discussed. The distribution law of the major metallic components, particularly V and Mn, of the acid leachate in organic phase, aqueous phase and precipitated phase were quantified. The effects of concentrations of vanadium and manganese, and the recycle of trioctylamine on the separation efficiency were elucidated. The techno-economic feasibility of the new process was discussed. The results show that a vanadium extraction rate of 99% can be achieved even for an acid leachate with high concentration of manganese. The vanadium extraction rate of 97% and manganese precipitation rate of 96% are maintained after 5 regeneration cycles. The new process is techno-economically applausive.
-
表 1 钒渣酸浸液的主要金属元素含量
Table 1. Content of the major metallic elements in acid leachate of vanadium slag
g/L V Mn Ca Al Mg Cr Si Ti 23.44 3.247 0.9646 0.0808 3.631 0.01135 0.0835 0.0325 表 2 酸浸液主要金属元素在有机萃取相和水相中的平衡浓度
Table 2. Equilibrium concentration of major metallic elements both in organic and aqueous phases
元素 各相平衡浓度/( g·L−1) 萃取率E/ % 分配系数 KD 选择性系数β 酸浸液 萃余水相 有机萃取相 V 23.44 0.0533 5.828 99.77 109.4 Mn 3.247 0.0686 0.000431 2.833 0.006 17403.6 Mg 3.516 3.134 0.0155 1.940 0.004946 22108.52 Ca 0.9646 0.5992 0.01145 7.10 0.01911 5722.14 表 3 有机胺萃取耦合CO2矿化过程沉淀的主要元素含量
Table 3. Content of the major metallic elements in the precipitation of the simultaneous amine extraction and CO2 mineralization process
% V Mn Ca Mg Cr Ti 0.6857 45.27 0.7574 0.4439 <0.01 <0.01 表 4 V2O5产品的化学成分
Table 4. Content of the major chemical compound in V2O5 product
% V2O5 Mn Ca Mg Al Ti Fe 99.56 0.034 0.2117 0.3286 <0.01 <0.01 <0.01 表 5 两种酸浸液中各金属元素含量
Table 5. Content of the major metallic elements in two kinds of acid leachate
g/L V Mn Ca Mg Al Cr Si Ti 酸浸液-1 23.44 3.247 0.9646 3.631 0.0808 0.0114 0.0835 0.0325 酸浸液-2 24.50 9.750 0.800 3.530 0.0338 0.0129 0.0289 0.0042 表 6 酸浸液浓度对钒锰分离的影响
Table 6. Effect of acid leachate concentration on the separation of V and Mn
V/Mn 钒的萃取率/ % 锰的萃取率/ % 锰的沉淀率/ % 钒的分配系数 钒锰的选择性系数 酸浸液-1 7.22 99.94 2.833 97.92 109.4 17403.6 酸浸液-2 2.51 99.76 37.86 59.59 75.78 20.43 -
[1] Li Peng, Xiang Guohong, Wang Yongjun, et al. A review on the application of vanadium[J]. Chemical Enterprise Management, 2021,(1):72−73. (李鹏, 向国洪, 王勇军, 等. 钒的应用研究综述[J]. 化工管理, 2021,(1):72−73. doi: 10.19900/j.cnki.ISSN1008-4800.2021.01.036Li Peng, Xiang Guohong, Wang Yongjun, et al. A review on the application of vanadium[J]. Chemical Enterprise Management, 2021(1): 72-73. doi: 10.19900/j.cnki.ISSN1008-4800.2021.01.036 [2] Yu Tangxia, Cao Jing, Wen Jing, et al. Selection of additives for vanadium slag calcification roasting and process optimization[J]. Journal of Materials and Metallurgy, 2020,19(3):176−184. (余唐霞, 曹婧, 温婧, 等. 钒渣钙化焙烧添加剂选择及工艺优化[J]. 材料与冶金学报, 2020,19(3):176−184.Yu Tangxia, Cao Jing, Wen Jing, et al. Selection of additives for vanadium slag calcification roasting and process optimization[J]. Journal of Materials and Metallurgy, 2020, 19(3): 176-184. [3] Aimbetova I, Jimenez-Castaneda R, Clavijo-Blanco J, et al. Investigation of optical and physico-chemical properties of titanium-doped V2O5 nanofilms[J]. Kompleksnoe Ispolzovanie Mineralnogo Syra, 2023,(2):47−52. [4] Lv Changxiao, Zhang Ting'an, Zhang Ying, et al. Comprehensive recovery of vanadium from calcification roasting-acid leaching tailings[J]. Chinese Journal of Rare Metals, 2020,44(11):1208−1214. (吕昌晓, 张廷安, 张莹, 等. 从钙化焙烧-酸浸尾渣中综合回收钒的研究[J]. 稀有金属, 2020,44(11):1208−1214.Lü Changxiao, Zhang Ting, an, Zhang Ying, et al. Comprehensive recovery of vanadium from calcification roasting-acid leaching tailings[J]. Chinese Journal of Rare Metals, 2020, 44(11): 1208-1214. [5] Yin Danfeng, Peng Yi, Sun Zhaohui, et al. Influencing factors of calcified roasting and thermal analysis to the process of vanadium slag produced from Pangang[J]. Metal Mine, 2012,(4):91−94. (尹丹凤, 彭毅, 孙朝晖, 等. 攀钢钒渣钙化焙烧影响因素研究及过程热分析[J]. 金属矿山, 2012,(4):91−94. doi: 10.3969/j.issn.1001-1250.2012.04.024Yin Danfeng, Peng Yi, Sun Zhaohui, et al. Influencing factors of calcified roasting and thermal analysis to the process of vanadium slag produced from Pangang[J]. Metal Mine, 2012(4): 91-94. doi: 10.3969/j.issn.1001-1250.2012.04.024 [6] Liu Zishuai, Zhang Yiming, Dai Zilin, et al. Coextraction of vanadium and manganese from high-manganese containing vanadium wastewater by a solvent extraction-precipitation process[J]. Frontiers of Chemical Science and Engineering, 2020,14(5):902−912. doi: 10.1007/s11705-019-1887-z [7] Cai Zhenlei, Feng Yali, Li Haoran, et al. Selective separation and extraction of vanadium(IV) and manganese(Ⅱ) from co-leaching solution of roasted stone coal and pyrolusite via solvent extraction[J]. Industrial & Engineering Chemistry Research, 2013,52(38):13768−13776. [8] 李道玉, 刘波, 陈婷. 去除含钒浸出液中锰的方法: 中国, CN110331298 A[P]. 2019-10-15.Li Daoyu, Liu Bo, Chen Ting. Methods for removing manganese from vanaium-containing leaching solution: China, CN110331298A[P]. 2019-10-15. [9] Mei Ying, Xue Yuhua, Ye Hengpeng, et al. Selective separation and recovery of manganese from manganese-bearing wastewater using carborn dioxide[J]. Journal of Chemical Industry and Engineering, 2017,68(7):2798−2804. (梅颖, 薛余化, 叶恒朋, 等. 利用二氧化碳选择性分离回收含锰废水中的锰[J]. 化工学报, 2017,68(7):2798−2804. doi: 10.11949/j.issn.0438-1157.20161737Mei Ying, Xue Yuhua, Ye Hengpeng, et al. Selective separation and recovery of manganese from manganese-bearing wastewater using carborn dioxide[J]. Journal of Chemical Industry and Engineering, 2017, 68(7): 2798-2804. doi: 10.11949/j.issn.0438-1157.20161737 [10] Bong-Yeolm Yeom, Lee Chang Soo, Hwang Taek-Sung. A new hybrid ion exchanger: Effect of system parameters on the adsorption of vanadium (V)[J]. Journal of Hazardous Materials, 2009,166(1):415−420. doi: 10.1016/j.jhazmat.2008.11.032 [11] Heo Seo-Jin, Jeon Jong-Hyuk, Kim Rina, et al. Separation of vanadium and tungsten from spent SCR DeNOX catalyst by ion-exchange column[J]. Resources Recycling, 2021,30(4):54−63. doi: 10.7844/kirr.2021.30.4.54 [12] Cai Zhenlei, Feng Yali, Zhou Yuzhao, et al. Selective separation and extraction of vanadium (V) over manganese (II) from co-leaching solution of roasted stone coal and pyrolusite using solvent extraction[J]. Jom, 2013,65(11):1492−1498. doi: 10.1007/s11837-013-0768-z [13] Kumar Jyothi Rajesh, Shin Shun Myung, Yoon Ho Sung, et al. Separation and recovery of vanadium from synthetic leach liquor solutions containing iron, calcium, sodium, aluminum, and manganese by the solvent extraction technique[J]. Separation Science and Technology, 2014,49(6):819−828. doi: 10.1080/01496395.2013.863925 [14] 刘长军, 胡可, 刘颖颖, 等. 一种有机胺萃取耦合CO2矿化分离钙化焙烧酸浸液中钒和/或锰的方法: CN115074529A[P]. 2022-09-20.Liu Changjun, Hu Ke, Liu Yingying, et al. A method for separating vanadium and/or manganese from calcified acid leaching solution by organic amine extraction coupled with CO2 mineralization: China, CN115074529A[P]. 2022-09-20. [15] Shen Biao. Temperature control of acid leaching process for calcified clinker of vanadium slag[J]. Iron Steel Vanadium Titanium, 2018,39(5):30−36. (申彪. 钒渣钙化焙烧熟料酸浸工艺温度控制[J]. 钢铁钒钛, 2018,39(5):30−36. doi: 10.7513/j.issn.1004-7638.2018.05.006Shen Biao. Temperature control of acid leaching process for calcified clinker of vanadium slag[J]. Iron Steel Vanadium Titanium, 2018, 39(5): 30-36. doi: 10.7513/j.issn.1004-7638.2018.05.006 [16] GB/T 8704.5-2020. 钒铁 钒含量的测定 硫酸亚铁铵滴定法和电位滴定法[S].GB/T 8704.5-2020. Ferrovanadium. Determination of vanadium content. The ammonium ferrous sulfate titrimetric method and the potentiometric titrimetric method[S]. [17] GB/T 1506-2002. 锰矿石 锰含量的测定 电位滴定法和硫酸亚铁铵滴定法[S].GB/T 1506-2002. Manganese ores. Determination of manganese content. Potentiometric method and ammonium iron(Ⅱ) sulphate titrimetric method[S]. [18] 周雪娇. V-S-H2O系E-pH图及V2O3溶解动力学研究[D]. 昆明: 昆明理工大学, 2010.Zhou Xuejiao. Pourbaix diagrams for V-S-H2O system and dissolution kinetics of vanadium trioxide[D]. Kunming: Kunming University of Science and Technology, 2010. [19] Zhou Xuejiao, Wei Chang, Li Xingbin, et al. Thermodynamics of vanadium−sulfur−water systems at 298 K[J]. Hydrometallurgy, 2011,106(1-2):104−112. doi: 10.1016/j.hydromet.2010.12.003 [20] Chen Bianfang, Huang Sheng, Liu Biao, et al. Thermodynamic analysis for separation of vanadium and chromium in V(IV)-Cr(III)-H2O system[J]. Transactions of Nonferrous Metals Society of China, 2018,28(3):567−573. doi: 10.1016/S1003-6326(18)64689-8 -





 下载:
下载: