Effect of glucose content on the lithium storage performance of Li3V2(PO4)3/C cathode materials prepared by sol-gel combustion method
-
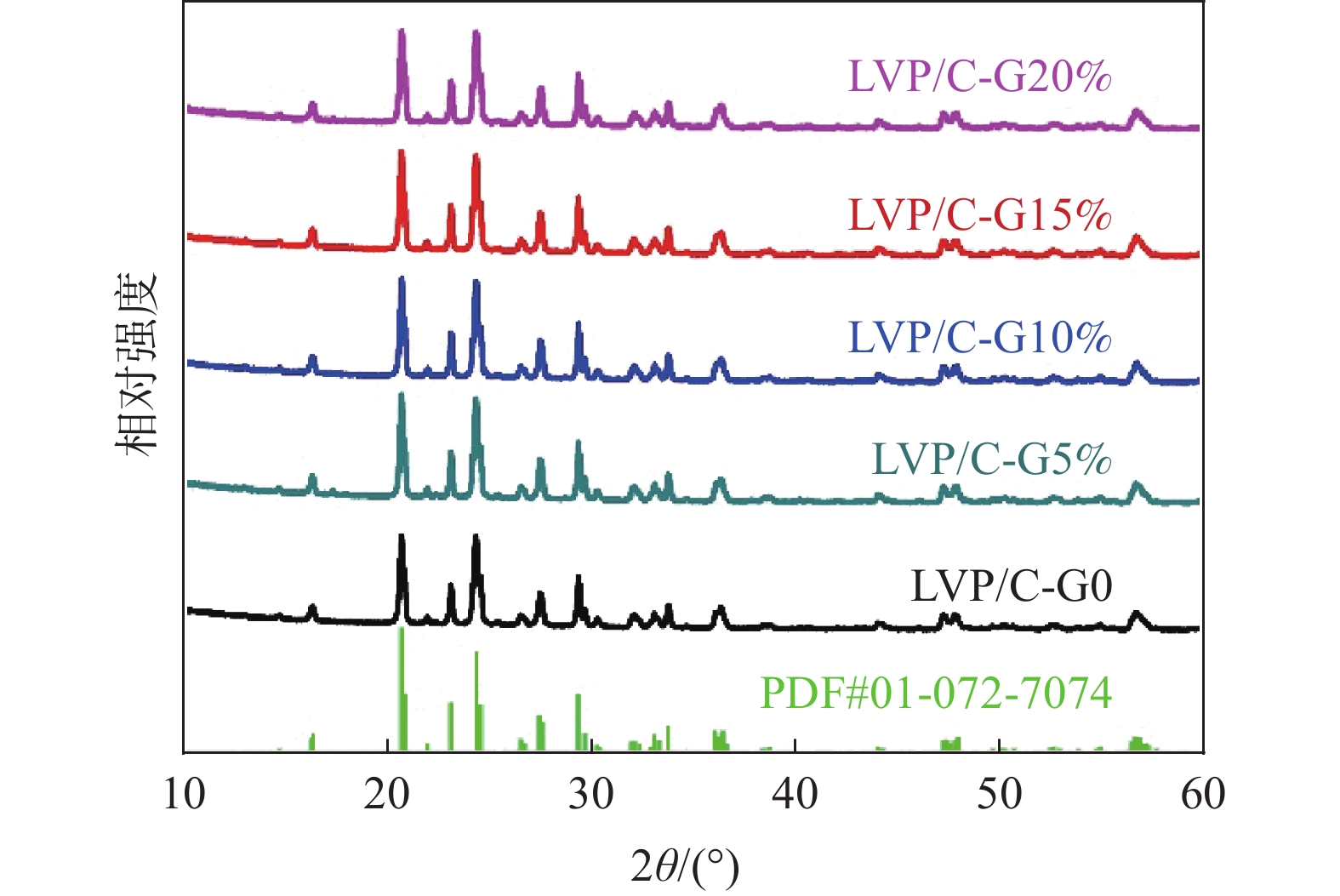
摘要: 通过改变葡萄糖添加量,采用改进的溶胶凝胶燃烧法成功制备出不同碳含量的亚微多孔Li3V2(PO4)3(LVP)/C复合材料。系统研究了葡萄糖添加量对LVP的结构、形貌及电化学性能的影响。添加葡萄糖虽然没有改变LVP的晶型结构和晶格参数,但是添加了葡萄糖的样品中出现了有利于电子传输和Li+扩散的纳米针状颗粒,且随着葡萄糖添加量的增加,纳米针状颗粒的体积分数增加,从而提高了LVP/C正极材料的倍率性能。葡萄糖碳化生成的无定形碳均匀包覆在LVP颗粒的表面,提高了复合材料的电导率,电导率随着葡萄糖添加量的增加而增加,但是葡萄糖添加量过多会导致碳包覆层过厚,不利于Li+的传输。得益于适当的葡萄糖添加量以及纳米针状颗粒和多孔结构,LVP/C-G15%样品具有优异的储锂性能,其在10 C的高倍率下循环200次后仍可提供75.1 mAh/g的放电比容量,容量保持率高达89.0%。
-
关键词:
- Li3V2(PO4)3/C正极材料 /
- 储锂性能 /
- 葡萄糖添加量 /
- 溶胶凝胶燃烧法 /
- 碳含量
Abstract: In this paper, submicrometer porous Li3V2(PO4)3 (LVP)/C composites with different carbon contents were successfully prepared by a modified sol-gel combustion method by changing the amount of glucose added. The effects of glucose addition on the structure, morphology and electrochemical properties of LVP were systematically studied. Although the addition of glucose did not change the crystal structure and lattice parameters of LVP, nanoneedle-like particles appeared in the samples with glucose, which were beneficial to electron transport and Li+ diffusion. With the increase of glucose content, the volume fraction of nanoneedle-like particles increased, thus improving the rate performance of LVP/C cathode materials. The amorphous carbon generated by carbonization of glucose is evenly coated on the surface of LVP particles, which improves the conductivity of the composites. The conductivity increases with the increase of glucose content. However, excessive glucose addition will lead to too thick carbon coating, which is not conducive to the transmission of Li+. Benefiting from the appropriate amount of glucose addition, nanoneedle-like particles and porous structure, LVP/C-G15% sample has excellent lithium storage performance. It can still provide a discharge specific capacity of 75.1 mAh/g after 200 cycles at a high rate of 10 C, and the capacity retention rate is as high as 89.0%. -
表 1 不同葡萄糖添加量的LVP/C样品的XRD-Rietveld精修晶体学参数
Table 1. Refined crystallographic parameters from XRD-Rietveld for LVP/C materials with different glucose additions
样品 a /nm b /nm c /nm Β/ (°) V /nm3 Rwp /% LVP/C-G0 0.8611 0.8605 1.205 90.56 0.8930 6.94 LVP/C-G5% 0.8610 0.8601 1.205 90.54 0.8925 7.06 LVP/C-G10% 0.8611 0.8602 1.205 90.55 0.8926 6.89 LVP/C-G15% 0.8610 0.8604 1.205 90.53 0.8928 6.68 LVP/C-G20% 0.8610 0.8603 1.205 90.54 0.8926 6.53 表 2 通过等效电路对EIS数据进行拟合得到的LVP/C-Gx (x = 0, 10%, 15%和20%)电极的动力学参数
Table 2. The kinetic parameters of LVP/C-Gx (x = 0, 10%, 15% and 20%) electrodes obtained by fitting the EIS data through the equivalent circuit
样品 Rs /Ω Rct /Ω σ /(Ω·s−1/2) LVP/C-G0 1.684 498.1 129.1 LVP/C-G10% 1.634 371.1 79.11 LVP/C-G15% 1.254 318.7 21.91 LVP/C-G20% 1.331 271.0 46.05 -
[1] Sun Chunwen, Rajasekhara Shreyas, Dong Youzhong, et al. Hydrothermal synthesis and electrochemical properties of Li3V2(PO4)3/C-based composites for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2011,3(9):3772−3776. [2] Membreño Nellymar, Park Kyusung, Goodenough John B, et al. Electrode/electrolyte interface of composite α-Li3V2(PO4)3 cathodes in a nonaqueous electrolyte for lithium ion batteries and the role of the carbon additive[J]. Chemistry of Materials, 2015,27(9):3332−3340. doi: 10.1021/acs.chemmater.5b00447 [3] Membreño Nellymar, Xiao Penghao, Park Kyu Sung, et al. In situ Raman study of phase stability of α-Li3V2(PO4)3 upon thermal and laser heating[J]. The Journal of Physical Chemistry C, 2013,117(23):11994−12002. doi: 10.1021/jp403282a [4] Yin S C, Strobel P S, Grondey H, et al. Li2.5V2(PO4)3: A room-temperature analogue to the fast-ion conducting high-temperature γ-phase of Li3V2(PO4)3[J]. Chemistry Of Materials, 2004,16(8):1456−1465. doi: 10.1021/cm034802f [5] Peng Yi, Tan Rou, Ma Jianmin, et al. Electrospun Li3V2(PO4)3 nanocubes/carbon nanofibers as free-standing cathodes for high-performance lithium-ion batteries[J]. Journal of Materials Chemistry A, 2019,7(24):14681−14688. doi: 10.1039/C9TA02740H [6] Tan Huiteng, Xu Lianhua, Geng Hongbo, et al. Nanostructured Li3V2(PO4)3 cathodes[J]. Small, 2018,14(21):1800567. doi: 10.1002/smll.201800567 [7] Cui Kai, Hu Shuchun, Li Yongkui. Nitrogen-doped graphene nanosheets decorated Li3V2(PO4)3/C nanocrystals as high-rate and ultralong cycle-life cathode for lithium-ion batteries[J]. Electrochimica Acta, 2016,210:45−52. doi: 10.1016/j.electacta.2016.05.099 [8] Mohanty Debabrata, Lu Zhenlun, Hung I Ming. Effect of carbon coating on electrochemical properties of Nitrogen-doped graphene cathode synthesized by citric-acid gel method for lithium-ion batteries[J]. Journal of Applied Electrochemistry, 2023,53(5):1003−1013. doi: 10.1007/s10800-022-01828-1 [9] Chen Jian, Zhao Na, Guo Feifan. Impact of carbon coating thickness on the electrochemical properties of Li3V2(PO4)3/C composites[J]. Russian Journal of Electrochemistry, 2017,53(4):339−344. doi: 10.1134/S102319351704005X [10] Zhou Ji, Sun Xinyu, Wang Kai. Preparation of high-voltage Li3V2(PO4)3 co-coated by carbon and Li7La3Zr2O12 as a stable cathode for lithium-ion batteries[J]. Ceramics International, 2016,42(8):10228−10236. doi: 10.1016/j.ceramint.2016.03.144 [11] Han Hui, Qiu Feng, Liu Zhentao, et al. ZrO2-coated Li3V2(PO4)3/C nanocomposite: A high-voltage cathode for rechargeable lithium-ion batteries with remarkable cycling performance[J]. Ceramics International, 2015,41(7):8779−8784. doi: 10.1016/j.ceramint.2015.03.103 [12] Liao Yuxing, Li Chao, Lou Xiaobing, et al. Carbon-coated Li3V2(PO4)3 derived from metal-organic framework as cathode for lithium-ion batteries with high stability[J]. Electrochimica Acta, 2018,271:608−616. doi: 10.1016/j.electacta.2018.03.100 [13] Chen Yueqian, Xiang Kaixiong, Zhu Yirong, et al. Porous, nitrogen-doped Li3V2(PO4)3/C cathode materials derived from oroxylum and their exceptional electrochemical properties in lithium-ion batteries[J]. Ceramics International, 2019,45(4):4980−4989. doi: 10.1016/j.ceramint.2018.11.198 [14] Sun Hongxia, Du Haoran, Yu Mengkang, et al. Vesicular Li3V2(PO4)3/C hollow mesoporous microspheres as an efficient cathode material for lithium-ion batteries[J]. Nano Research, 2019,12(8):1937−1942. doi: 10.1007/s12274-019-2461-1 [15] Lee Hwang Sheng, Ramar Vishwanathan, Kuppan Saravanan, et al. Key design considerations for synthesis of mesoporous α-Li3V2(PO4)3/C for high power lithium batteries[J]. Electrochimica Acta, 2021,372:137831. doi: 10.1016/j.electacta.2021.137831 [16] Zhang Le, Xiang Hongfa, Li Zhong, et al. Porous Li3V2(PO4)3/C cathode with extremely high-rate capacity prepared by a sol-gel-combustion method for fast charging and discharging[J]. Journal of Power Sources, 2012,203:121−125. doi: 10.1016/j.jpowsour.2011.11.082 [17] Ou Qingzhu, Tang Yan, Zhong Yanjun, et al. Submicrometer porous Li3V2(PO4)3/C composites with high rate electrochemical performance prepared by sol-gel combustion method[J]. Electrochimica Acta, 2014,137:489−496. doi: 10.1016/j.electacta.2014.04.178 [18] Taddesse Paulos, Belete Birhanu. Substitutional effect on structural, electrical and electrochemical behaviors of LiMn1.977(Ce, Cu)0.023O4 nanoparticles prepared by sol-gel combustion method[J]. Chemical Physics, 2019,522:260−266. doi: 10.1016/j.chemphys.2019.03.015 [19] Li Nali, Tong Yanwei, Yi Dawei, et al. Facile synthesis of Li3V2(PO4)3/C composite with a complex morphology and its excellent electrochemical performance as cathode material for lithium ion batteries[J]. Materials Research Express, 2019,6(11):115530. doi: 10.1088/2053-1591/ab49c1 [20] Li Nali, Yu Yong, Tong Yanwei, et al. Sc3+-doping effects on porous Li3V2(PO4)3/C cathode with superior rate performance and cyclic stability[J]. Ceramics International, 2021,47(24):34218−34224. doi: 10.1016/j.ceramint.2021.08.331 [21] Li Nali, Tong Yanwei, Yi Dawei, et al. Effect of Zr4+ doping on the morphological features and electrochemical performance of monoclinic Li3V2(PO4)3/C cathode material synthesized by an improved sol-gel combustion technique[J]. Journal of Alloys and Compounds, 2021,868:158771. doi: 10.1016/j.jallcom.2021.158771 [22] Li Ruhong, Liu Jianchao, Chen Tianrui, et al. Systematic evaluation of lithium-excess polyanionic compounds as multi-electron reaction cathodes[J]. Nanoscale, 2019,11(36):16991−17003. doi: 10.1039/C9NR05751J [23] Yu Shicheng, Mertens Andreas, Kungl Hans, et al. Morphology dependency of Li3V2(PO4)3/C cathode material regarding to rate capability and cycle life in lithium-ion batteries[J]. Electrochimica Acta, 2017,232:310−322. doi: 10.1016/j.electacta.2017.02.136 [24] Chen Lin, Yan Bo, Xu Jing, et al. Bicontinuous structure of Li3V2(PO4)3 clustered via carbon nanofiber as high-performance cathode material of Li-ion batteries[J]. ACS Applied Materials & Interfaces, 2015,7(25):13934−13943. [25] Yu Shicheng, Mertens Andreas, Schierholz Roland, et al. An advanced all phosphate lithium-ion battery providing high electrochemical stability, high rate capability and long-term cycling performance[J]. Journal of the Electrochemical Society, 2017,164:A370−A379. doi: 10.1149/2.1151702jes [26] Xiong Fangyu, Tan Shuangshuang, Wei Qiulong, et al. Three-dimensional graphene frameworks wrapped Li3V2(PO4)3 with reversible topotactic sodium-ion storage[J]. Nano Energy, 2017,32:347−352. doi: 10.1016/j.nanoen.2016.12.050 [27] Guo Shuainan, Bai Ying, Geng Zhenfeng, et al. Facile synthesis of Li3V2(PO4)3 cathode material for lithium-ion battery via freeze-drying[J]. Journal of Energy Chemistry, 2019,32:159−165. doi: 10.1016/j.jechem.2018.07.011 [28] Rui Xianhong, Yan Qingyu, Skyllas Kazacos Maria, et al. Li3V2(PO4)3 cathode materials for lithium-ion batteries: A review[J]. Journal of Power Sources, 2014,258:19−38. doi: 10.1016/j.jpowsour.2014.01.126 [29] Oh Woong, Park Hyunyoung, Jin Bong-Soo, et al. Understanding the structural phase transitions in lithium vanadium phosphate cathodes for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2020,8(20):10331−10336. doi: 10.1039/C9TA12435G [30] Ruan Tingting, Lu Shengli, Lu Junyang, et al. Unraveling the intercalation chemistry of multi-electron reaction for polyanionic cathode Li3V2(PO4)3[J]. Energy Storage Materials, 2023,55:546−555. doi: 10.1016/j.ensm.2022.12.021 [31] Bi Linnan, Song Zhicui, Liu Xiaoqin, et al. Critical roles of RuO2 nano-particles in enhancing cyclic and rate performance of Lisicon Li3V2(PO4)3 cathode materials[J]. Journal of Alloys and Compounds, 2020,845:156271. doi: 10.1016/j.jallcom.2020.156271 [32] Zhang Shu, Gu Qin, Tan Shan, et al. Improved electrochemical properties of the Li3V2(PO4)3 cathode material synthesized from a V(III) precursor[J]. Journal of Alloys and Compounds, 2019,802:583−590. doi: 10.1016/j.jallcom.2019.06.240 -





 下载:
下载: