Preparation and properties of lithium titanate anode materials with different titanium sources
-
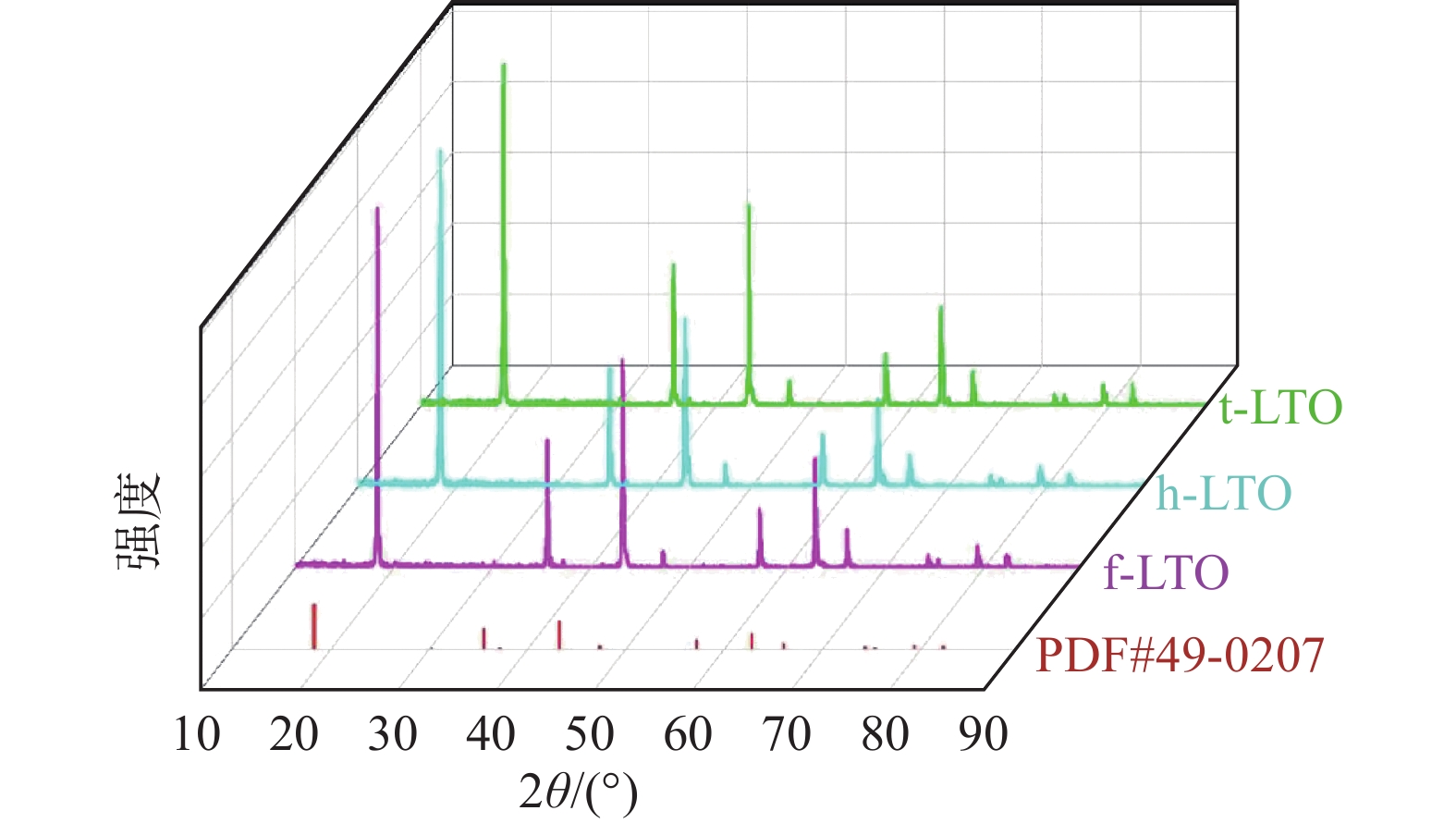
摘要: 通过高温固相法制备球形Li4Ti5O12负极材料,探究了采用不同钛源所制备的Li4Ti5O12的性能差异。通过XRD、SEM对制备的材料进行结构及形貌的表征,同时将合成的材料进行电化学测试。结果表明,分别以纳米TiO2(P40)、工业H2TiO3、含铁工业H2TiO3为钛源制备的Li4Ti5O12材料t-LTO、h-LTO、f-LTO在较低倍率0.2 C下的放电比容量分别为170.0、156.3、150.7 mAh/g,在较高倍率5 C下的放电比容量分别为91.9、93.0、26.7 mAh/g。在1 C下充放电循环100次后容量保持率分别为97.4%、97.3%、94.6%。以纳米TiO2及工业H2TiO3为钛源制备得到的Li4Ti5O12具有较好的电化学性能,因此可用较为纯净的工业H2TiO3为钛源代替价格高昂的TiO2制备Li4Ti5O12负极材料。Abstract: Spherical Li4Ti5O12 anode materials were prepared by high temperature solid phase method, and the effects of different titanium sources on the properties of Li4Ti5O12 anode materials were investigated. The structure and morphology of the prepared materials were characterized by XRD and SEM, and the synthesized materials were tested by electrochemical method. The results show that the discharge specific capacities of t-LTO, h-LTO and f-LTO prepared with nano TiO2(P40), industrial H2TiO3 and industrial H2TiO3 containing iron are 170.0, 156.3 and 150.7 mAh/g at lower charge/discharge rate of 0.2 C, respectively. And the discharge specific capacities at the higher rate of 5 C are 91.9, 93.0 and 26.7 mAh/g, respectively. After 100 cycles of charging and discharging at 1 C, the capacity retention rates are 97.4%, 97.3% and 94.6%, respectively. Li4Ti5O12 prepared with nano TiO2 and industrial H2TiO3 has good electrochemical performance, so relatively pure industrial H2TiO3 can be used as a substitute for expensive TiO2 to prepare Li4Ti5O12 anode materials.
-
图 3 (a)t-LTO、h-LTO和f-LTO在不同速率下的倍率性能; (b) t-LTO、h-LTO和f-LTO在1 C下的循环性能;(c) t-LTO、h-LTO和f-LTO在0.5 mV/s下的CV曲线; (d) t-LTO、h-LTO和f-LTO的EIS曲线
Figure 3. (a) Magnification performance diagrams of t-LTO, h-LTO and f-LTO at different rates, (b) Cycle performance diagrams of t-LTO, h-LTO and f-LTO at 1 C, (c) CV curves of t-LTO, h-LTO and f-LTO at 0.5 mV/s and (d) EIS curves of t-LTO, h-LTO and f-LTO
-
[1] Li Chunxiao. Research progress of anode materials for lithium ion batteries[J]. Advanced Materials Industry, 2017,(9):27−33. (李春晓. 锂离子电池负极材料研究进展[J]. 新材料产业, 2017,(9):27−33.Li Chunxiao. Research progress of anode materials for lithium ion batteries[J]. Advanced Materials Industry, 2017(9): 27-33. [2] Li Wang, Liu Jiali. Preparation and electrode formulations of lithium titanate[J]. Iron Steel Vanadium Titanium, 2019,40(2):50−54. (李旺, 刘佳丽. 钛酸锂材料的制备及电极配方研究[J]. 钢铁钒钛, 2019,40(2):50−54. doi: 10.7513/j.issn.1004-7638.2019.02.008Li Wang, Liu Jiali. Preparation and electrode formulations of lithium titanate[J]. Iron Steel Vanadium Titanium, 2019, 40(2): 50-54. doi: 10.7513/j.issn.1004-7638.2019.02.008 [3] Shi Ying, Wen Lei, Wu Minjie, et al. Applications of the carbon materials on lithium titanium oxide as anode for lithium ion batteries[J]. Progress In Chemistry, 2017,29(1):149−161. (石颖, 闻雷, 吴敏杰, 等. 碳材料在钛酸锂负极材料中的应用[J]. 化学进展, 2017,29(1):149−161.Shi Ying, Wen Lei, Wu Minjie, et al. Applications of the carbon materials on lithium titanium oxide as anode for lithium ion batteries[J]. Progress In Chemistry, 2017, 29(1): 149-161. [4] Li Wang, Liu Jiali, Zhou Lan. Dynamic and frontier of modifications of Li4Ti5O12 anode material[J]. Iron Steel Vanadium Titanium, 2018,39(4):11−16. (李旺, 刘佳丽, 周兰. 国内外钛酸锂负极材料改性研究动态与前沿[J]. 钢铁钒钛, 2018,39(4):11−16.Li Wang, Liu Jiali, Zhou Lan. Dynamic and frontier of modifications of Li4Ti5O12 anode material[J]. Iron Steel Vanadium Titanium, 2018, 39(4): 11-16. [5] Meng Weiwei. Preparation of Li4Ti5O12/C composite and its application to anode material of high rate lithium-ion battery[J]. Iron Steel Vanadium Titanium, 2017,38(3):52−56,77. (孟伟巍. 改性钛酸锂复合材料作为高倍率锂离子电池负极材料研究[J]. 钢铁钒钛, 2017,38(3):52−56,77. doi: 10.7513/j.issn.1004-7638.2017.03.009Meng Weiwei. Preparation of Li4Ti5O12 /C composite and its application to anode material of high rate lithium-ion battery[J]. Iron Steel Vanadium Titanium, 2017, 38(3): 52-56, 77. doi: 10.7513/j.issn.1004-7638.2017.03.009 [6] Ma Guangqiang. Lithium ion battery cathode material Li4Ti5O12 prepared by high-temperature solid method using metatitanic acid[J]. Electronic Components and Materials, 2016,35(3):19−21. (马光强. 偏钛酸高温固相法制备锂离子电池负极材料尖晶石Li4Ti5O12[J]. 电子元件与材料, 2016,35(3):19−21.Ma Guangqiang. Lithium ion battery cathode material Li4Ti5O12 prepared by high-temperature solid method using metatitanic acid[J]. Electronic Components And Materials, 2016, 35(3): 19-21. [7] Yu Xiaolin, Wu Xianming, Li Yehua, et al. Preparation and electrochemical performance of Li4Ti5O12 anode material by high-temperature solid-state method[J]. Guangdong Chemical Industry, 2017,44(7):61−63. (于小林, 吴显明, 李叶华, 等. 高温固相法合成Li4Ti5O12及其电化学性能研究[J]. 广东化工, 2017,44(7):61−63. doi: 10.3969/j.issn.1007-1865.2017.07.028Yu Xiaolin, Wu Xianming, Li Yehua, et al. Preparation and electrochemical performance of Li4Ti5O12 anode material by high-temperature solid-state method[J]. Guangdong Chemical Industry, 2017, 44(7): 61-63. doi: 10.3969/j.issn.1007-1865.2017.07.028 [8] Liu Wei, Zhang Ni, Bai Yang, et al. Synthesis of lithium-ion battery anode material Li4Ti5O12 by the microwave assisted sol–gel method[J]. Journal of the Chinese Ceramic Society, 2010,38(12):2279−2283. (刘微, 张妮, 白阳, 等. 微波辅助溶胶-凝胶法合成锂离子电池负极材料Li4Ti5O12[J]. 硅酸盐学报, 2010,38(12):2279−2283.Liu Wei, Zhang Ni, Bai Yang, et al. Synthesis of lithium-ion battery anode material Li4Ti5O12 by the microwave assisted sol–gel method[J]. Journal of the Chinese Ceramic Society, 2010, 38(12): 2279-2283. [9] Lian Tianrou, Lu Yuxiao, Wu Bing, et al. Preparation and transport property of nano-Li4Ti5O12 anode materials[J]. Journal of Materials Engineering, 2021,49(3):59−66. (廉恬柔, 卢玉晓, 吴冰, 等. 纳米级Li4Ti5O12负极材料的制备及其输运特性[J]. 材料工程, 2021,49(3):59−66.Lian Tianrou, Lu Yuxiao, Wu Bing, et al. Preparation and transport property of nano-Li4Ti5O12 anode materials[J]. Journal of Materials Engineering, 2021, 49(3): 59-66. [10] You Yahua, Zhang Yang, Li Xueliang, et al. Hydrothermal synthesis and electrochemical properties of Li4Ti5O12 anode material[J]. Metallic Functional Materials, 2013,20(3):28−31. (尤亚华, 张杨, 李学良, 等. 水热法制备Li4Ti5O12负极材料及其电化学性能[J]. 金属功能材料, 2013,20(3):28−31.You Yahua, Zhang Yang, Li Xueliang, et al. Hydrothermal synthesis and electrochemical properties of Li4Ti5O12 anode material[J]. Metallic Functional Materials, 2013, 20(3): 28-31. [11] Yu Xiaolin, Wu Xianming, Ding Xinxiong, et al. Preparation and electrochemical performance of Li4Ti5O12 nanosheets by hydrothermal method[J]. Modern Chemical Industry, 2018,38(2):83−86. (于小林, 吴显明, 丁心雄, 等. 水热法制备纳米片钛酸锂及其性质研究[J]. 现代化工, 2018,38(2):83−86. doi: 10.16606/j.cnki.issn0253-4320.2018.02.019Yu Xiaolin, Wu Xianming, Ding Xinxiong, et al. Preparation and electrochemical performance of Li4Ti5O12 nanosheets by hydrothermal method[J]. Modern Chemical Industry, 2018, 38(2): 83-86. doi: 10.16606/j.cnki.issn0253-4320.2018.02.019 [12] Lv Jing, Zuo Yuxiang, Huang Bing, et al. Synthesis and properties of lithium titanate anode material prepared by different sizes of titanium dioxide[J]. Chemical Engineer, 2016,30(7):84−85. (吕婧, 左玉香, 黄兵, 等. 不同粒径的纳米二氧化钛合成钛酸锂负极材料[J]. 化学工程师, 2016,30(7):84−85. doi: 10.16247/j.cnki.23-1171/tq.20160784Lv Jing, Zuo Yuxiang, Huang Bing, et al. Synthesis and properties of lithium titanate anode material prepared by different sizes of titanium dioxide[J]. Chemical Engineer, 2016, 30(7): 84-85. doi: 10.16247/j.cnki.23-1171/tq.20160784 [13] 周若. 钛酸锂的制备及改性研究[D]. 唐山: 华北理工大学, 2020.Zhou Ruo. Preparation and modification of lithium titanate[D]. Tangshan: North China University of Science and Technology, 2020. [14] Yuan T, Tan Z, Ma C, et al. Challenges of spinel Li4Ti5O12 for lithium‐ion battery industrial applications[J]. Advanced Energy Materials, 2017,7(12):1601625. doi: 10.1002/aenm.201601625 -





 下载:
下载: