Thermodynamic analysis of the effect of oxygenation on the low-temperature chlorination selectivity of carbonized slag
-
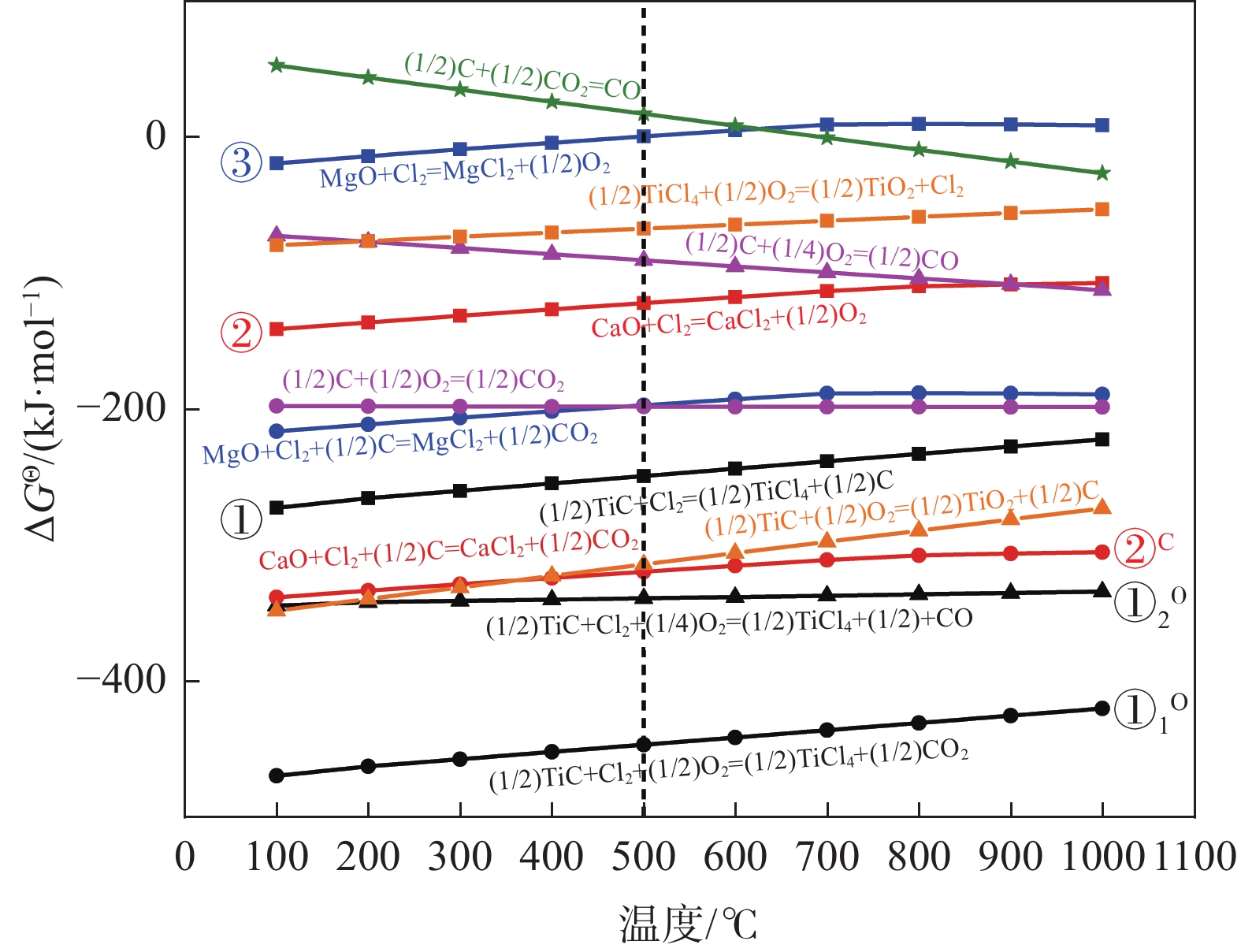
摘要: 利用Factsage热力学软件及数据库,计算分析了引入氧气对碳化钛渣中TiC和钙镁氧化物氯化的影响,以及加氧选择性强化碳化渣中TiC氯化反应的同时减少或抑制钙镁氧化物氯化反应的调控方案。结果表明,加氧低温氯化可促进渣中TiC的氯化反应进程,提高TiC的氯化率;当低温氯化反应温度为500 ℃时,在Cl2/TiC摩尔比为1.80~3.50范围内,MgO不发生氯化反应,氧气的引入可以使CaO氯化反应受到抑制,降低CaO氯化率;氯氧气体摩尔比为4.00:1.00,氯化反应使碳化渣中TiC质量分数减小至4.02%时,可将氯化气体切换为氯气与氮气的混合气体,进一步氯化渣中残留的TiC直至2.50%以下;此加氧分步氯化与不加氧直接氯化相比,氯气总消耗量可减少25.69%,CaO总氯化率可减少37.74%。Abstract: Based on the Factsage thermodynamic software and database, the effects of introducing oxygen on the TiC and calcium magnesium oxide chlorination in titanium carbide slag are analyzed, and the regulatory scheme of adding oxygen to selectively enhance the chlorination reaction of TiC in the slag while reducing or inhibiting the chlorination reaction of calcium and magnesium oxides are studied. The results show that the oxygenated low-temperature chlorination can promote the chlorination process and increase the chlorination rate of TiC in the slag. When the low-temperature chlorination reaction temperature is 500 ℃ and the molar ratio of Cl2/TiC is 1.80~3.50, MgO does not chlorinate, and the introduction of oxygen can inhibit the CaO chlorination reaction and reduce the CaO chlorination rate. When the molar ratio of chlorine-oxygen gas is 4.00:1.00, and the mass fraction of TiC with chlorination reaction in carbonization slag is reduced to 4.02%, the chlorinated gas can be switched to a mixture of chlorine gas and nitrogen gas to further chlorinate the residual TiC in the slag to less than 2.50%. Compared with direct chlorination without adding oxygen, the total chlorine consumption can be reduced by 25.69% and the total chlorination rate of CaO can be reduced by 37.74%.
-
表 1 碳化渣的主要成分及含量
Table 1. Main components and content of carbonized slag
成分 质量分数/% 摩尔数/mol* 摩尔比* TiC 13.80 0.23 1.00 CaO 26.57 0.47 2.04 MgO 8.09 0.20 0.87 SiO2 25.25 0.42 1.83 Al2O3 14.23 0.14 0.61 MnO 0.55 0.01 0.04 Fe 1.00 0.02 0.09 V2O5 0.24 0.01 0.04 TiO2 3.60 0.05 0.22 FeO 2.00 0.03 0.13 C 4.00 0.33 1.43 其它 0.67 *:以100 g碳化渣计的摩尔数和以TiC=1.00 mol为基折算的摩尔比。 表 2 TiC-2.04CaO-0.87MgO-nCl2-mO2反应体系中各物相氯化率随O2含量变化规律
Table 2. The variation of chlorination rates of various phases with O2 contents in the TiC-2.04CaO-0.87MgO-nCl2-mO2 reaction system
Cl2含量n TiC氯化率/% CaO氯化率/% 最优O2/TiC 摩尔比 Cl2/O2摩尔比 未引入氧气 引入氧气 氯化率变化情况 未引入氧气 引入氧气 氯化率变化情况 1.80 51.35 100.00 +48.65 50.33 0.00 −50.33 1.10 1.64 1.90 54.20 100.00 +45.80 53.12 0.00 −53.12 1.10 1.73 2.00 57.06 100.00 +42.94 55.88 0.49 −55.39 1.00 2.00 2.10 59.91 100.00 +40.09 58.82 5.39 −53.43 1.00 2.10 2.20 62.76 100.00 +37.24 61.27 10.29 −50.98 0.90 2.44 2.30 65.62 100.00 +34.38 64.22 15.20 −49.02 0.90 2.56 2.40 68.47 100.00 +31.53 67.16 20.10 −47.06 0.80 3.00 2.50 71.32 100.00 +28.68 70.10 25.00 −45.10 0.80 3.13 2.70 77.03 100.00 +22.97 75.49 34.80 −40.69 0.70 3.86 2.90 82.74 100.00 +17.26 80.88 44.61 −36.27 0.60 4.83 3.10 88.44 100.00 +11.56 86.76 54.41 −32.35 0.50 6.20 3.30 94.15 100.00 +5.85 92.16 64.22 −27.94 0.40 8.25 3.50 100.00 100.00 +0.00 98.04 74.02 −24.02 0.30 11.67 表 3 碳化渣加氧氯化不同阶段组分含量变化(初始渣质量以100 g计)
Table 3. Changes in component of carbonized slag during different stages of oxychlorination. (The initial slag mass is calculated as 100 g)
氯化反
应阶段Cl2/O2
摩尔比混合气体
中Cl2含量/
molTiC初
始量/gTiC初始
量/molTiC残
余量/gTiC残余
量/molCaO初
始量/gCaO初始
量/molCaO残
余量/gCaO残余
量/mol其他组分
含量/gTiC质量
分数/%TiC总氯
化率%CaO总氯
化率/%第一阶段 4.00 13.80 0.23 3.93 0.07 26.57 0.47 18.76 0.33 59.63 4.02 71.50 29.39 第二阶段 0.00 3.93 0.07 3.93 0.07 18.76 0.33 18.76 0.33 59.63 4.02 71.50 29.39 0.02 3.59 0.06 18.11 0.32 3.67 73.99 31.84 0.06 2.99 0.05 16.81 0.30 3.05 78.33 36.73 0.10 2.40 0.04 15.35 0.27 2.41 82.61 42.23 0.14 1.80 0.03 14.21 0.25 1.80 86.96 46.52 表 4 碳化渣直接氯化不同阶段组分含量变化(初始渣质量以100 g计)
Table 4. Changes in composition and content of slag before and after the first stage chlorination reaction without introducing oxygen. (The initial slag mass is calculated as 100 g)
氯化反
应阶段混合气体中
Cl2含量/molTiC初
始量/gTiC初始
量/molTiC残
余量/gTiC残余
量/molCaO初
始量/gCaO初始
量/molCaO残
余量/gCaO残余
量/mol其他组分
含量/gTiC质量
分数/%TiC总氯
化率%CaO总
氯化率/%第一阶段 13.80 0.23 3.93 0.07 26.57 0.47 7.94 0.14 59.63 3.63 71.50 70.11 第二阶段 0.00 3.93 0.07 3.93 0.07 7.94 0.14 7.94 0.14 59.63 3.63 71.50 70.11 0.02 3.59 0.06 7.37 0.13 3.31 73.99 72.26 0.06 2.99 0.05 6.24 0.11 2.75 78.33 76.51 0.10 2.40 0.04 4.54 0.08 2.18 82.61 82.91 0.14 1.80 0.03 3.40 0.06 1.63 86.96 87.20 -
[1] Ahmadi E, Rezan S A, Baharun N, et al. Chlorination kinetics of titanium nitride for production of titanium tetrachloride from nitrided ilmenite[J]. Metallurgical and Materials Transactions B, 2017,48(5):2354−2366. doi: 10.1007/s11663-017-1011-z [2] Yang F, Wen L Y, Yue D, et al. Study on reaction behaviors and mechanisms of rutile TiO2 with different carbon addition in fluidized chlorination[J]. Journal of Materials Research and Technology, 2022,18:1205−1217. doi: 10.1016/j.jmrt.2022.02.131 [3] Zhu F X, Qiu K H, Sun Z H. Preparation of titanium from TiCl4 in a molten fluoride-chloride salt[J]. Electrochemistry, 2017,85(11):715−720. doi: 10.5796/electrochemistry.85.715 [4] Shi J J, Qiu Y C, Yu B, et al. Titanium extraction from titania-bearing blast furnace slag: A review[J]. American Journal of Respiratory and Critical Care Medicine, 2022,74(2):654−667. [5] Qin J, Wang Y, You Z X, et al. Carbonization and nitridation of vanadium–bearing titanomagnetite during carbothermal reduction with coal[J]. Journal of Materials Research and Technology, 2020,9(3):4272−4282. doi: 10.1016/j.jmrt.2020.02.053 [6] Peng Yi. Thermodynamic analysis on the selective chlorination of carbonized Pangang BF slag at low temperature[J]. Titanium Industry Progress, 2005,6:45−49. (彭毅. 碳化攀钢高炉渣低温选择氯化的热力学分析[J]. 钛工业进展, 2005,6:45−49. doi: 10.3969/j.issn.1009-9964.2005.01.013Peng Yi. Thermodynamic analysis on the selective chlorination of carbonized Pangang BF slag at low temperature [J]. Titanium Industry Progress, 2005, 6: 45-49. doi: 10.3969/j.issn.1009-9964.2005.01.013 [7] 刘晓华. 改性含钛高炉渣高温碳化低温氯化的研究[D]. 沈阳: 东北大学, 2009.Liu Xiaohua. Study on high-temperature carbonization and low-temperature chlorination on modified titanium bearing blast furnace slag[D]. Shengyang: Northeastern University, 2009. [8] Taki T, Komoto S, Otomura K, et al. Chloride pyrometallurgy of uranium ore (II)[J]. Journal of Nuclear Science and Technology, 1996,33(4):327−332. doi: 10.1080/18811248.1996.9731912 [9] Hiroyuki M, Fumitaka T. Chlorination kinetics of ZnO with Ar-Cl2-O2 gas and the effect of oxychloride formation[J]. Metallurgical and Materials Transactions B, 2006,37(3):413−420. doi: 10.1007/s11663-006-0026-7 [10] Hiroyuki M, Tasuku H, Fumitaka T. Chlorination kinetics of ZnFe2O4 with Ar-Cl2-O2 gas[J]. Materials Transactions, 2006,47(10):2524−2532. doi: 10.2320/matertrans.47.2524 [11] Jungshin K, Toru H O. Removal of iron from titanium ore by selective chlorination using TiCl4 under high oxygen chemical potential[J]. International Journal of Mineral Processing, 2016,149:111−118. doi: 10.1016/j.minpro.2016.02.014 -





 下载:
下载: