Study on concentration process of titanium white waste acid by sulfuric acid method
-
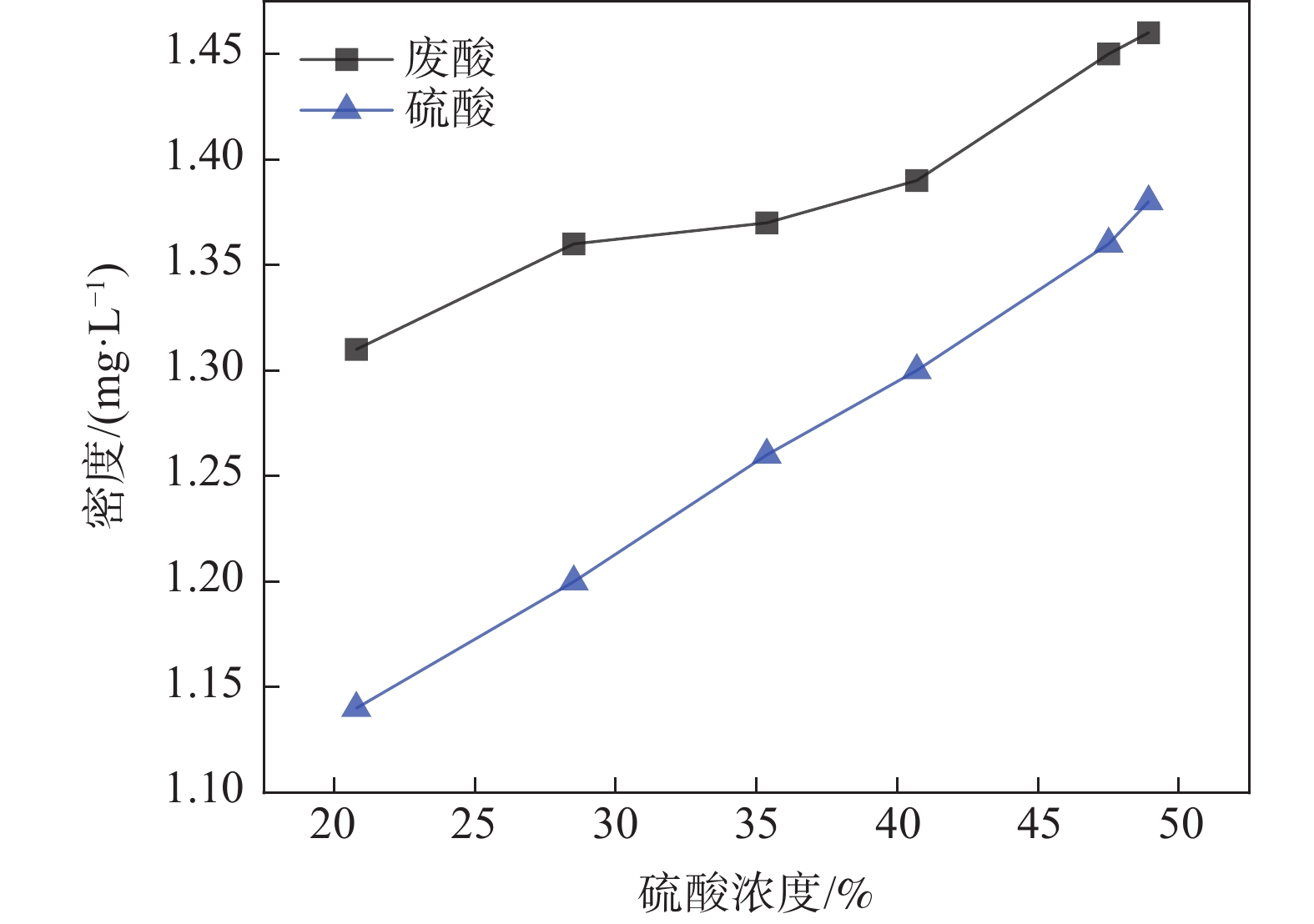
摘要: 为了提升废酸浓缩效率,以现场废酸为原料,在实验室采用真空旋转蒸发仪模拟现场废酸真空蒸发浓缩,开展废酸浓缩工艺单因素试验及正交试验,然后以实验室研究结果为依据,在现场开展了废酸浓缩工艺优化工业试验。结果表明,在相同的硫酸浓度条件下,废酸相对纯硫酸密度大,且随着硫酸浓度的增加,两者密度差逐渐减少;随着废酸浓缩时间、浓缩温度及浓缩真空度的增加,废酸浓缩浓度均逐渐增加,影响废酸浓缩浓度的关键影响因素为浓缩时间、浓缩真空度、浓缩时间×浓缩真空度、浓缩温度,重要度排序为浓缩时间>浓缩真空度>浓缩真空度×浓缩时间>浓缩温度。工业试验结果表明,当废酸浓缩时间、浓缩温度及浓缩真空度分别由2.06 h、108 ℃及30 kPa提升至2.68 h、112 ℃及45 kPa时,一级浓缩酸浓度均值由29.5%提升至38.5%。研究结果为硫酸法钛白生产企业提升废酸浓缩效率提供了重要的数据及理论支撑。Abstract: In order to improve the efficiency of waste acid concentration, the single factor test and orthogonal test of waste acid concentration process were carried out in the laboratory by using vacuum rotary evaporator to simulate the vacuum evaporation concentration of waste acid. Then, based on the results of laboratory research, the industrial test of waste acid concentration process optimization was carried out in the field. The results show that the density of waste acid is larger than that of pure sulfuric acid under the same sulfuric acid concentration, and the density difference between them decreases with the increase of sulfuric acid concentration. With the increase of concentration time, temperature and vacuum degree of waste acid, the concentration of waste acid increases gradually. The key influencing factors affecting the concentration of waste acid are concentration time, concentration vacuum degree, concentration time ×concentration vacuum degree and concentration temperature. The order of importance is concentration time > concentration vacuum degree > concentration vacuum degree × concentration time > concentration temperature. The industrial test results show that when the concentration time, concentration temperature and concentration vacuum degree of waste acid increase from 2.06 h, 108 °C and 30 kPa to 2.68 h, 112 °C and 45 kPa, respectively, the average concentration of primary concentrated acid increases from 29.5% to 38.5%. The research results provide important data and theoretical support for improving the concentration efficiency of waste acid in titanium dioxide production enterprises by sulfuric acid method.
-
表 1 废酸主要化学成分
Table 1. Main chemical components of waste acid
g/L H2SO4 CaSO4 FeSO4 TiOSO4 MgSO4 MnSO4 Al2(SO4)3 H2SiO3 245.00 1.26 126.29 10.76 6.36 0.64 9.99 1.64 表 2 废酸浓缩工艺正交试验设计及结果
Table 2. Orthogonal experimental design and results of waste acid concentration process
标准序 运行序 中心点 区组 温度/ ℃ 负压/kPa 时间/min 酸浓度/% 9 1 0 1 108 52.5 20 30.79 1 2 1 1 103 40 10 24.25 8 3 1 1 113 65 30 53.32 5 4 1 1 103 40 30 26.63 6 5 1 1 113 40 30 32.78 2 6 1 1 113 40 10 25.42 7 7 1 1 103 65 30 40.58 4 8 1 1 113 65 10 29.33 10 9 0 1 108 52.5 20 31.09 3 10 1 1 103 65 10 26.7 表 3 工业试验结果
Table 3. Industrial test results
No. t/h T/℃ P/kPa 一级酸浓度/% 测试值 平均 优化前 1-1 2.06 108 30 29.0 29.5 1-2 30.2 1-3 29.4 优化后 2-1 2.68 112 45 37.8 38.5 2-2 39.2 2-3 38.6 -
[1] Karimia L, Yazdanshenas M E, Khajavi R, et al. Optimizing the photocatalytic properties and the synergistic effects of graphene and nano titanium dioxide immobilized on cotton fabric[J]. Applied Surface Science, 2015,332:665−673. doi: 10.1016/j.apsusc.2015.01.184 [2] Romanovska N I, Manoryk P A, Ermokhina N I, et al. Effect of structural and dimensional characteristics of TiO2 and its photocatalytic activity in the oxidation of tetracycline[J]. Theoretical and Experimental Chemistry, 2019,55(5):345−353. doi: 10.1007/s11237-019-09627-0 [3] Sobczyk-guzenda Anna, Szymanski Witold, Jedrzejczak Anna, et al. Bactericidal and photowetting effects of titanium dioxide coatings doped with iron and copper/fluorine deposited on stainless steel substrates[J]. Surface & Coatings Technology, 2018,347:66−75. [4] Matsukura A, Onoda H. Influences of additives on phosphoric acid treatment of titanium dioxide as a novel white pigment[J]. Journal of Advanced Ceramics, 2015,4(3):211−216. doi: 10.1007/s40145-015-0151-3 [5] Kang J, Okabe T H. Removal of iron from titanium ore by selective chlorination using TiCl4 under oxygen content atmosphere[J]. International Journal of Mineral Processing, 2016,149:111−118. doi: 10.1016/j.minpro.2016.02.014 [6] Bi Sheng. Basic situation and development prospect of titanium dioxide industry in China in recent years[J]. Iron Steel Vanadium Titanium, 2021,42(2):1−4. (毕胜. 近年中国钛白粉行业基本状况及发展展望[J]. 钢铁钒钛, 2021,42(2):1−4.Bi Sheng. Basic situation and development prospect of titanium dioxide industry in China in recent years [J]. Iron Steel Vanadium Titanium , 2021, 42 (2) : 1-4. [7] Wei Q F, Ren X L, Guo J J, et al. Recovery and separation of sulfuric acid and iron from dilute acidic sulfate effluent and waste sulfuric acid by solvent extraction and stripping[J]. Journal of Hazardous Materials, 2016,304:1−9. doi: 10.1016/j.jhazmat.2015.10.049 [8] 毕胜. 2022年中国钛白粉行业发展及分析[J]. 钢铁钒钛, 2023, 44(1): 1-3.Bi Sheng. Development and analysis on 2022 titanium dioxide industry in China[J]. Iron Steel Vanadium Titanium, 2023, 44(1): 1-3. [9] 朱家骅, 叶世超, 夏素兰. 化工原理 [M]. 北京: 科学出版社, 2005.Zhu Jiahua, Ye Shichao, Xia Sulan. Principles of chemical engineering [M]. Beijing: Science Press, 2005. [10] 项双龙, 吴有丽, 唐明亮, 等. 750 kt/a湿法磷酸二级浓缩工艺研究 [J]. 硫磷设计与粉体工程, 2017 (4): 46-47.Xiang Shuanglong, Wu Youli, Tang Minglaing, et al. Research on secondary concentration process of 750 kt/a wet-process phosphoric acid [J]. Thiophosphate Design and Powder Engineering, 2017 (4): 46-47. [11] Nian Meiling, Ruan Qi, Jiang Hao, et al. Energy-efficient heat pump cocurrent multi-effect evaporation system for concentrated sucrose juice[J]. Journal of Food and Biotechnology, 2015,34(3):274−282. (念美玲, 阮奇, 江浩, 等. 浓缩蔗糖汁的高效节能热泵并流多效蒸发系统[J]. 食品与生物技术学报, 2015,34(3):274−282.Nian Meiling, Ruan Qi, Jiang Hao, et al. Energy-efficient heat pump cocurrent multi-effect evaporation system for concentrated sucrose juice [J]. Journal of Food and Biotechnology, 2015, 34 (3): 274-282. [12] 朱自强, 徐汛合. 化工热力学(第二版) [M]. 北京: 化学工业出版社, 1991.Zhu Ziqiang, Xu Xunhe. Chemical engineering thermodynamics ( Second edition ) [M]. Beijing: Chemical Industry Press, 1991. [13] Wang Haibo, Wang Kui, Sun Ke, et al. Cause analysis of blockage in heat exchanger of titanium dioxide waste acid concentration by sulfuric acid method[J]. Iron Steel Vanadium Titanium, 2021,42(5):115−125. (王海波, 王奎, 孙科, 等. 硫酸法钛白废酸浓缩换热器堵塞成因分析[J]. 钢铁钒钛, 2021,42(5):115−125.Wang Haibo, Wang Kui, Sun Ke, et al. Cause analysis of blockage in heat exchanger of titanium dioxide waste acid concentration by sulfuric acid method [J]. Iron Steel Vanadium Titanium, 2021, 42 (5): 115-125. -




 下载:
下载: