Leaching behavior of vanadium-chromium residue enhanced by ultrasonic
-
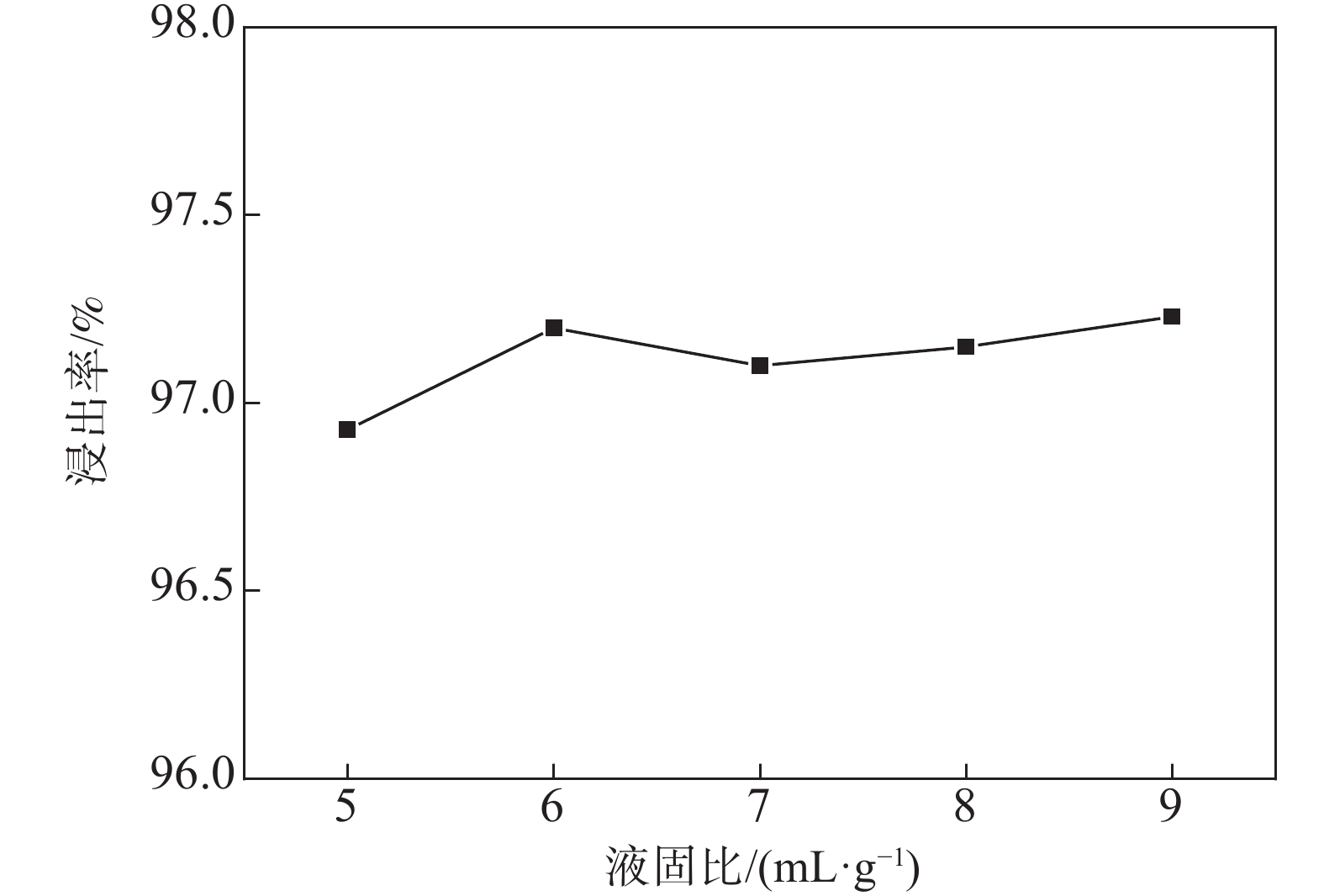
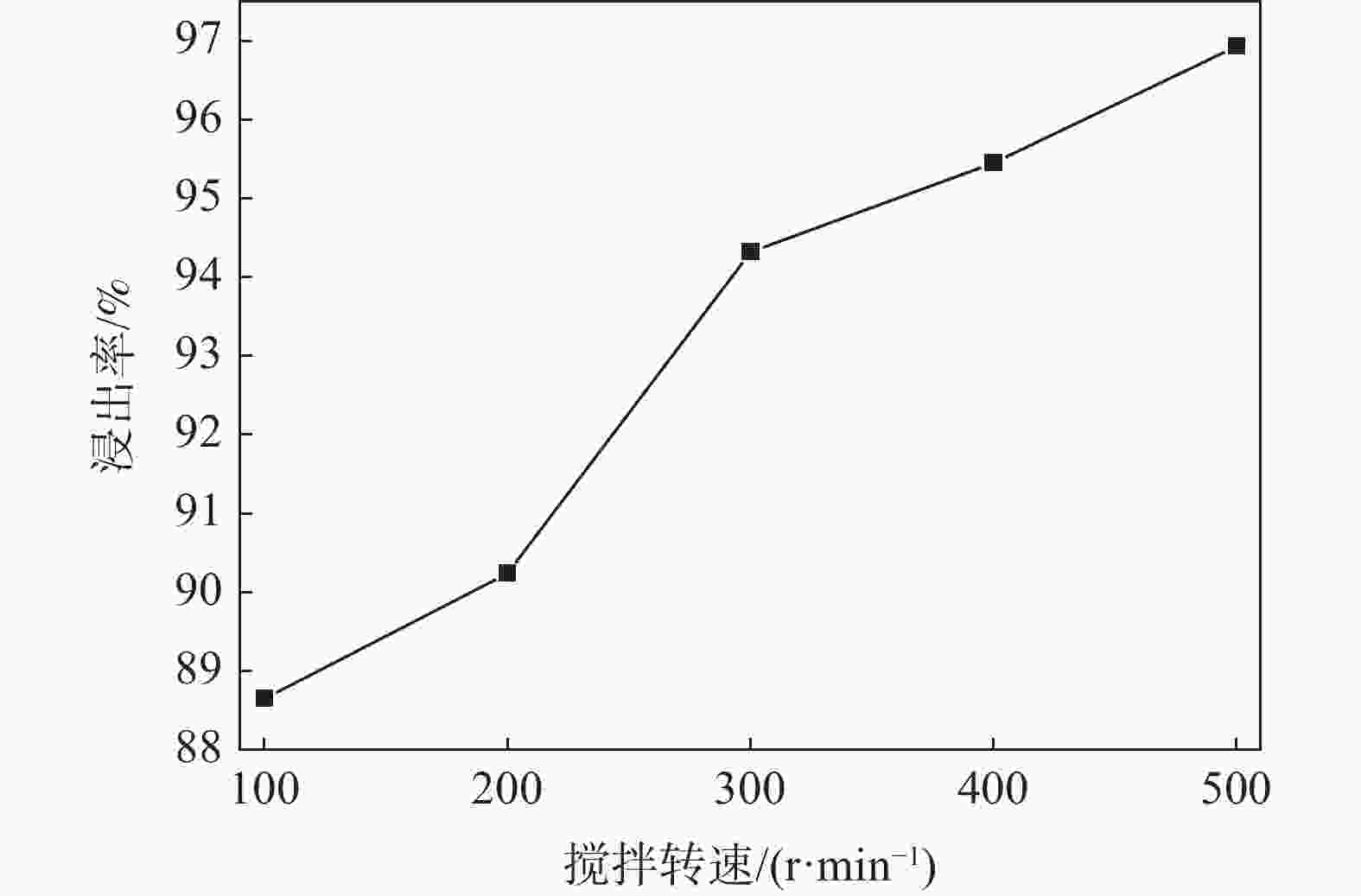
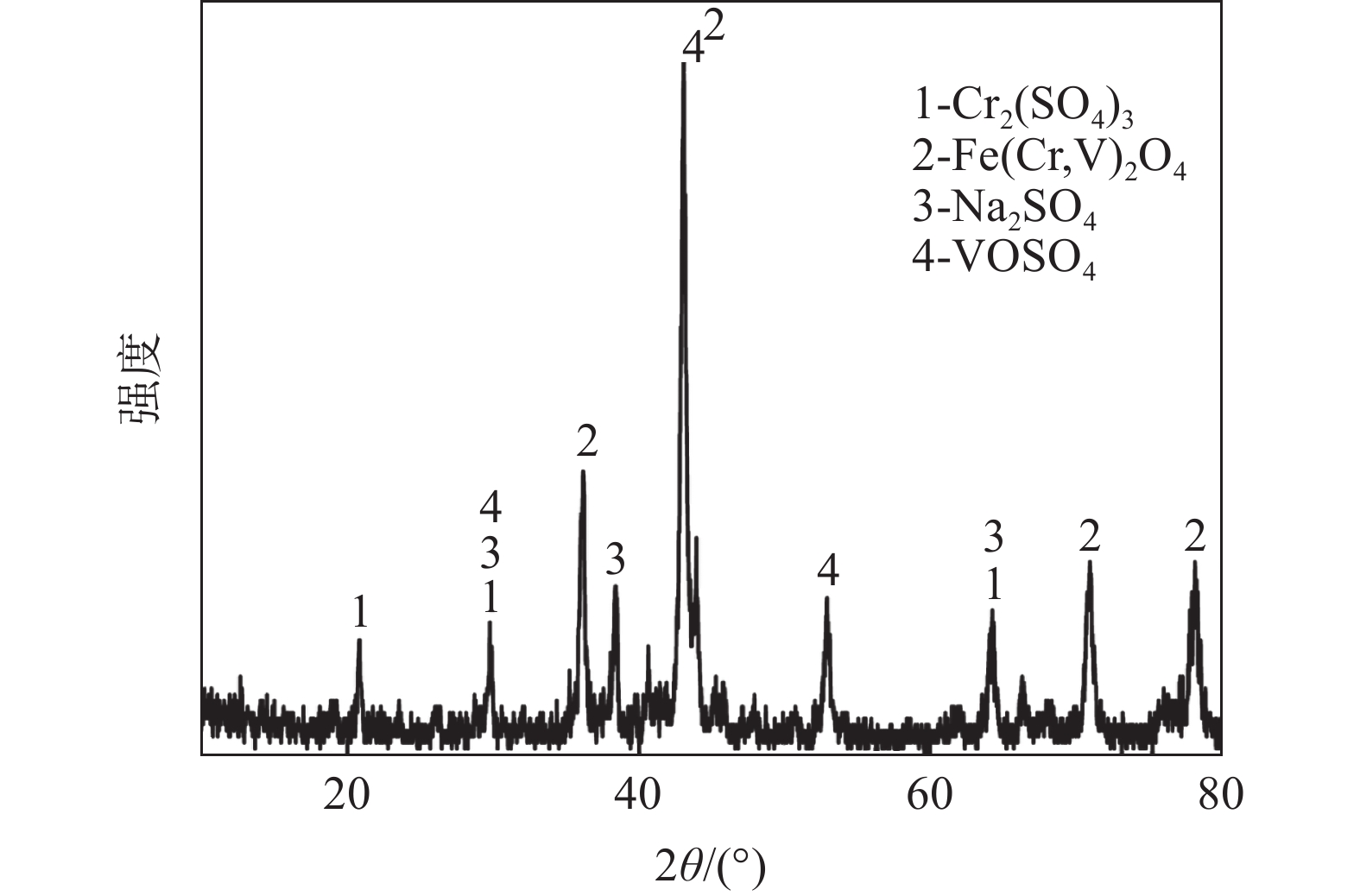
摘要: 钒铬滤饼中钒主要以低价态的形式存在,在碱性条件下难以直接溶出,通过焙烧-浸出的方式进行回收会带来较大能耗。引入超声对钒铬滤饼的碱性湿法浸出过程进行强化,实现低价钒向高价钒的氧化转化,可提高钒的浸出率。试验研究了氢氧化钠用量、反应温度、反应时间、反应液固比和搅拌转速等参数对钒浸出率的影响,结果表明,引入超声技术可以有效强化钒铬滤饼的湿法浸出过程,提高钒的浸出率。与直接碱性浸出相比,超声强化浸出过程可以使钒的浸出率提高34个百分点。在最佳反应条件下(氢氧化钠用量为m(NaOH)/m(钒铬滤饼)=0.5 g/g,反应温度为90 ℃,搅拌转速为500 r/min,反应时间为60 min,液固比为5 mL/g,超声频率为40 kHz),钒的浸出率可达96.9%。Abstract: Vanadium is existed as low-valence in the vanadium-chromium residue, it is hard to leach out in alkaline medium, high-energy consumption is accompanied by roasting-leaching process. In this paper, ultrasonic was introduced to enhance the leaching process of vanadium-chromium residue to oxidize the low valence vanadium to high valence and make contribute to high leaching efficiency. The effects of NaOH dosage, reaction temperature, reaction time, liquid-to-solid ratio and stirring rate on the leaching efficiency of vanadium were investigated. The results show that the leaching process is significantly enhanced by ultrasonic leaching and the leaching efficiency of vanadium is improved. Compared with direct alkaline leaching, the leaching efficiency of vanadium is increased about 34 percentage. The leaching rate of vanadium is up to 96.9% under the optimal conditions including the dosage of NaOH at m(NaOH)/(residue)=0.5 g/g, reaction temperature of 90 ℃, stirring rate at 500 r/min, reaction time of 60 min, liquid-to-solid ratio at 5 mL/g and ultrasonic frequency at 40 kHz.
-
表 1 钒铬滤饼的主要化学组成
Table 1. XRF results of main composition in vanadium-chromium residue
% O Cr Si Na S V Ca Cl Fe K Mg 41.09 14.36 12.02 9.76 12.02 1.63 1.42 4.09 0.33 0.29 0.20 -
[1] 杨守志. 钒冶金 [M]. 北京: 冶金工业出版社, 2010.Yang Shouzhi. Vanadium [M]. Beijing: Metallurgical Industry Press, 2010. [2] Wang Xuewen, Gao Daxiong, Chen Bianfang, et al. A clean metallurgical process for separation and recovery of vanadium and chromium from V-Cr-bearing reducing slag[J]. Hydrometallurgy, 2018,181:1−6. doi: 10.1016/j.hydromet.2018.08.008 [3] Hu Pengcheng, Zhang Yimin, Liu Tao, et al. Source separation of vanadium over iron from roasted vanadium bearing shale during acid leaching via ferric fluoride surface coating[J]. Journal of Cleaner Production, 2018,181:399−407. doi: 10.1016/j.jclepro.2018.01.226 [4] Fang Dean, Liao Xiang, Zhang Xuefei, et al. A novel resource utilization of the calcium-based semi-dry flue gas desulfurization ash: As a reductant to remove chromium and vanadium from vanadium industrial wastewater[J]. Journal of Hazardous Materials, 2018,342:436−445. doi: 10.1016/j.jhazmat.2017.08.060 [5] Wen Jing, Jiang Tao, Xu Yingzhe, et al. Efficient extraction and separation of vanadium and chromium in high chromium vanadium slag by sodium salt roasting-(NH4)2SO4 leaching[J]. Journal of Industrial and Engineering Chemistry, 2018,71:327−325. [6] Zhang Juhua, Zhang Wei, Xue Zhengliang. Oxidation kinetics of vanadium slag roasting in the presence of calcium oxide[J]. Mineral Processing and Extractive Metallurgy Review, 2017,38:265−273. doi: 10.1080/08827508.2017.1289197 [7] Wen Jing, Jiang Tao, Zhou Wanying, et al. A cleaner and efficient process for extraction of vanadium from high chromium vanadium slag: Leaching in (NH4)2SO4-H2SO4 synergistic system and NH4+ recycle[J]. Separation and Purification Technology, 2019,216:126−135. doi: 10.1016/j.seppur.2019.01.078 [8] Xiang Junyi, Huang Qingyun, Lü Xuewei, et al. Extraction of vanadium from converter slag by two-step sulfuric acid leaching process[J]. Journal of Cleaner Production, 2018,170:1089−1101. doi: 10.1016/j.jclepro.2017.09.255 [9] Peng Hao, Shang Qian, Chen Ronghua, et al. Oxidative leaching kinetic of vanadium from vanadium-chromium reducing residue by K2Cr2O7[J]. ACS Omega, 2020,5:8777−8783. doi: 10.1021/acsomega.0c00339 [10] Peng Hao, Yang Liu, Chen Ya, et al. A novel technology for recovery and separation of vanadium and chromium from vanadium-chromium reducing residue[J]. Applied Sciences, 2020,10:198. [11] Peng Hao, Guo Jing, Li Gang, et al. Leaching of vanadium and chromium from vanadium-chromium residue intensified with H2O2[J]. Iron Steel Vanadium Titanium, 2018,39(4):24−29. (彭浩, 郭静, 李港, 等. H2O2强化钒铬还原渣中钒和铬的浸出[J]. 钢铁钒钛, 2018,39(4):24−29.Peng Hao, Guo Jing, Li Gang, et al. Leaching of vanadium and chromium from vanadium-chromium residue intensified with H2O2 [J]. Iron Steel Vanadium Titanium, 2018, 39(4): 24-29. [12] Peng Hao, Liu Zuohua, Tao Changyuan. Selective leaching of vanadium from chromium residue intensified by electric field[J]. Journal of Environmental Chemical Engineering, 2015,3:1252−1257. doi: 10.1016/j.jece.2015.03.031 [13] Wang Zhonghang, Zheng Shili, Wang Shaona, et al. Electrochemical decomposition of vanadium slag in concentrated NaOH solution[J]. Hydrometallurgy, 2015,151:51−55. doi: 10.1016/j.hydromet.2014.10.017 [14] Liu Huibin, Du Hao, Wang Dawei, et al. Kinetics analysis of decomposition of vanadium slag by KOH sub-molten salt method[J]. Transactions of Nonferrous Metals Society of China, 2013,23:1489−1500. doi: 10.1016/S1003-6326(13)62621-7 [15] Liu Biao, Du Hao, Wang Shaona, et al. A novel method to extract vanadium and chromium from vanadium slag using molten NaOH-NaNO3 binary[J]. AIChE Journal, 2013,59:541−552. doi: 10.1002/aic.13819 [16] Liao Xueqin, Wang Nan, Hu Rong, et al. Effect of different ultrasound treatment time on physicochemical and structural properties of lotus root starch[J]. Food Science, 2023,(7):1−12. (廖雪勤, 汪楠, 胡荣, 等. 不同超声处理时间对莲藕淀粉理化和结构特性的影响[J]. 食品科学, 2023,(7):1−12.Liao Xueqin, Wang Nan, Hu Rong, et al. Effect of different ultrasound treatment time on physicochemical and structural properties of lotus root starch [J]. Food Science, 2023(7): 1-12. [17] Niu Fusheng, Bu Ziheng, Zhang Jinxia, et al. Mechanisms and kinetics of ultrasonic enhanced leaching of zinc-containing dust[J]. Nonferrous Metals (Extractive Metallurgy), 2023,7:15−21. (牛福生, 卜梓恒, 张晋霞, 等. 超声强化含锌尘泥浸出机理及动力学[J]. 有色金属(冶炼部分), 2023,7:15−21.Niu Fusheng, Bu Ziheng, Zhang Jinxia, et al. Mechanisms and kinetics of ultrasonic enhanced leaching of zinc-containing dust [J]. Nonferrous Metals (Extractive Metallurgy), 2023, 7: 15-21. [18] Liu Jianping, Chen Lin, Yuwen Chao, et al. Enhancement of leaching of Ca from steelmaking slag by ultrasonic for CO2 mineral sequestration[J]. Iron Steel Vanadium Titanium, 2022,43(1):91−98. (刘建平, 陈林, 宇文超, 等. 超声波增强炼钢渣中钙的浸出用于CO2矿物封存[J]. 钢铁钒钛, 2022,43(1):91−98.Liu Jianping, Chen Lin, Yuwen Chao, et al. Enhancement of leaching of Ca from steelmaking slag by ultrasonic for CO2 mineral sequestration[J]. Iron Steel Vanadium Titanium, 2022, 43(1): 91-98. [19] Guo Dongdong, Qin Qingwei, Chen Honghai, et al. Study on hydrometallurgical desulfurization for pressure leaching residue from copper smelting dust[J]. Nonferrous Metals (Extractive Metallurgy), 2021,12:105−111. (郭冬冬, 秦庆伟, 谌宏海, 等. 铜冶炼烟尘加压浸出渣湿法脱硫研究[J]. 有色金属(冶炼部分), 2021,12:105−111.Guo Dongdong, Qin Qingwei, Chen Honghai, et al. Study on hydrometallurgical desulfurization for pressure leaching residue from copper smelting dust [J]. Nonferrous Metals (Extractive Metallurgy), 2021, 12: 105-111. [20] Zhang Menglei, Song Hao, Zheng Chenghang, et al. Ultrasound assisted enhancement in leaching of valuable metals from spent hydrogenation catalyst[J]. Applied Chemical Industry, 2021,50:1747−1750. (张孟磊, 宋浩, 郑成航, 等. 超声强化浸出废加氢催化剂中的有价金属研究[J]. 应用化工, 2021,50:1747−1750. doi: 10.3969/j.issn.1671-3206.2021.07.001Zhang Menglei, Song Hao, Zheng Chenghang, et al. Ultrasound assisted enhancement in leaching of valuable metals from spent hydrogenation catalyst [J]. Applied Chemical Industry, 2021, 50: 1747-1750. doi: 10.3969/j.issn.1671-3206.2021.07.001 [21] Peng Hao, Liu Zuohua, Tao Changyuan. Leaching kinetics of vanadium with electro-oxidation and H2O2 in alkaline medium[J]. Energy & Fuels, 2016,30:7802−7807. -





 下载:
下载: