A new process for preparing Ti-Al alloys from low-valance titanium chlorides slurry by direct electrochemical reduction
-
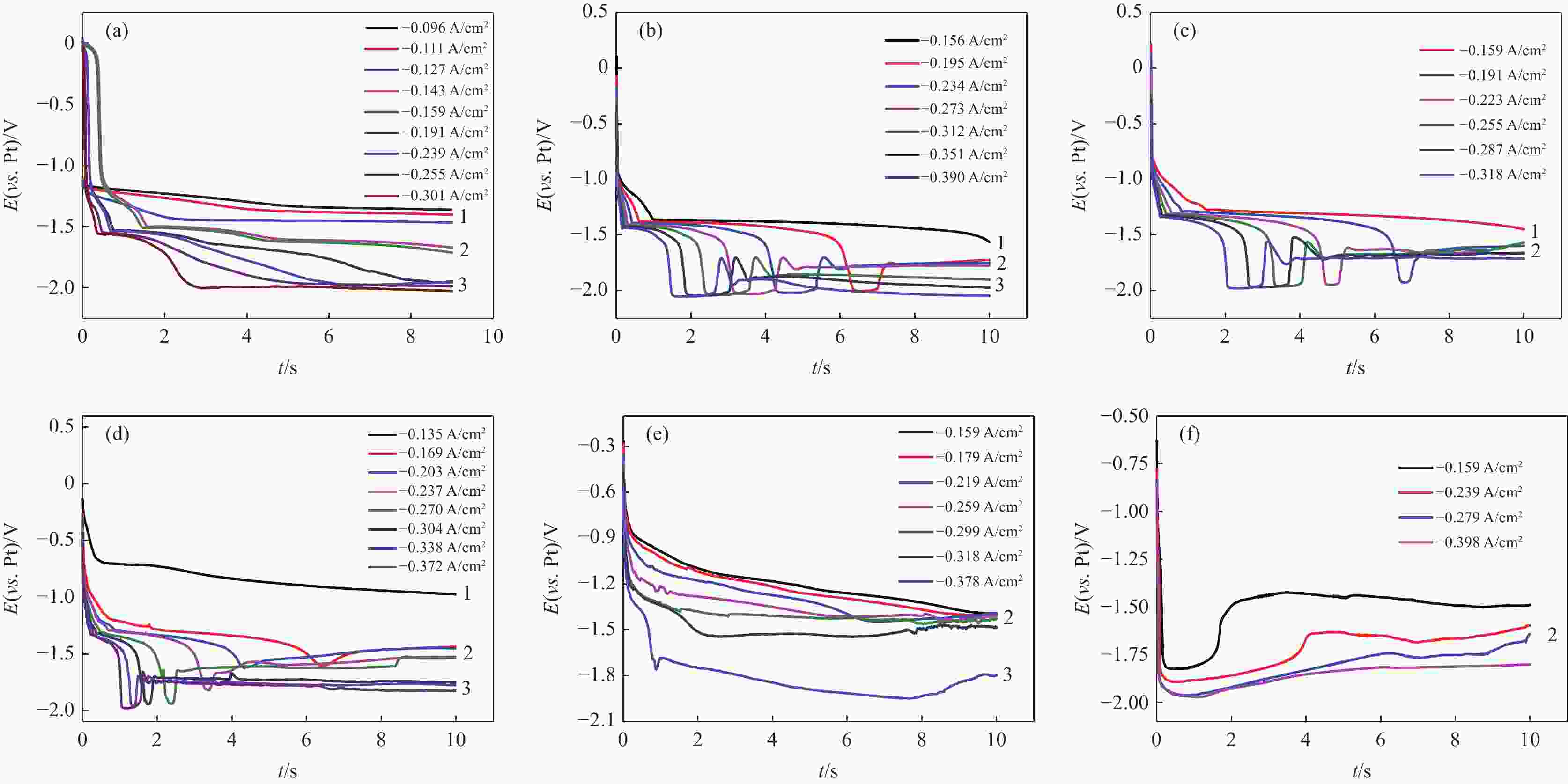
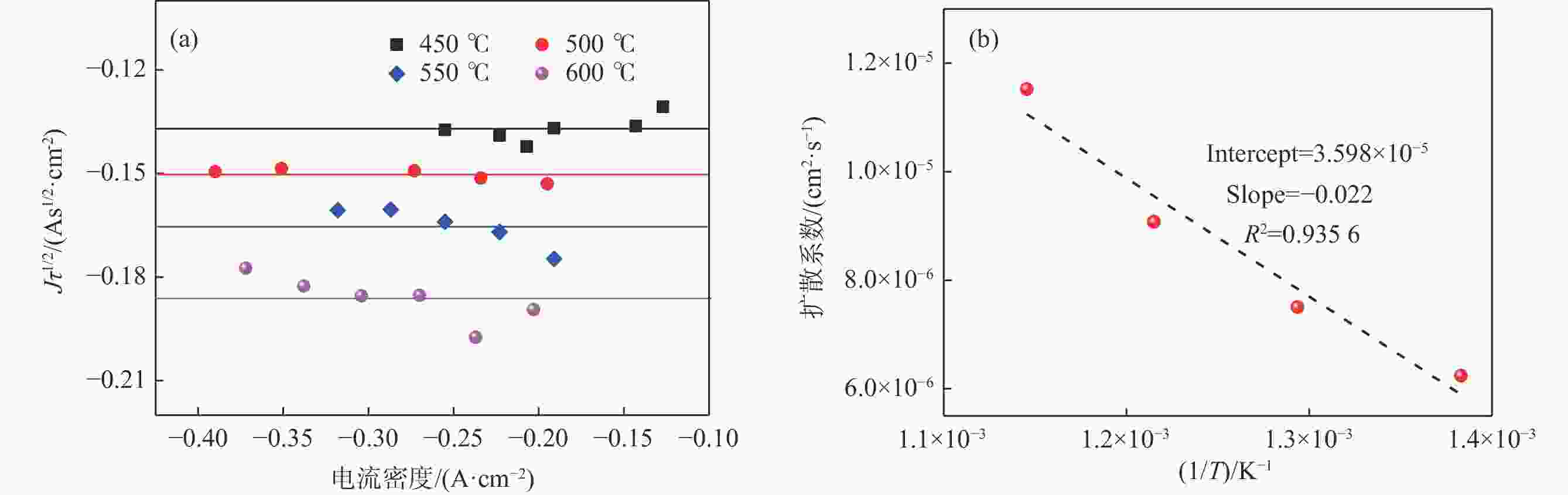
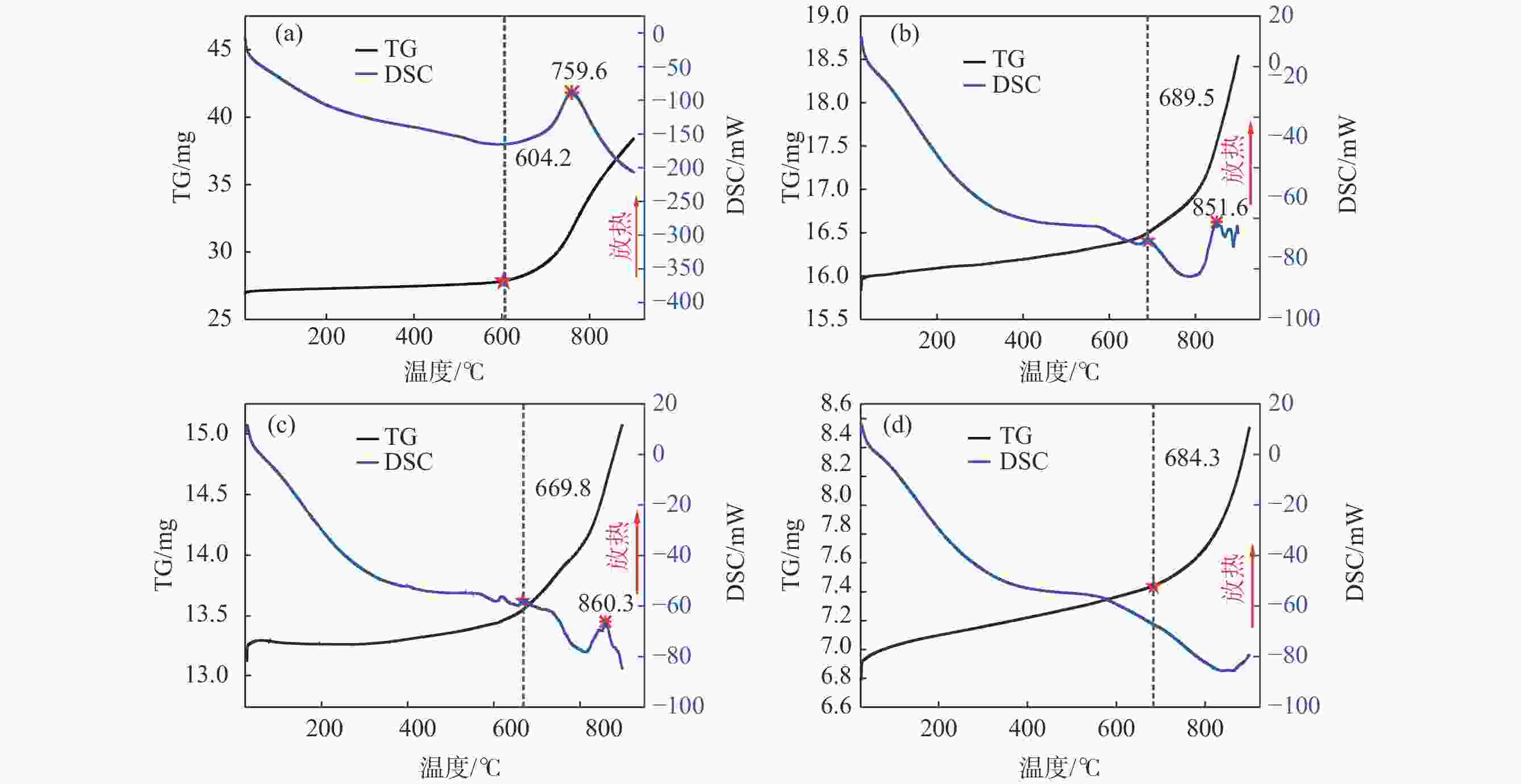
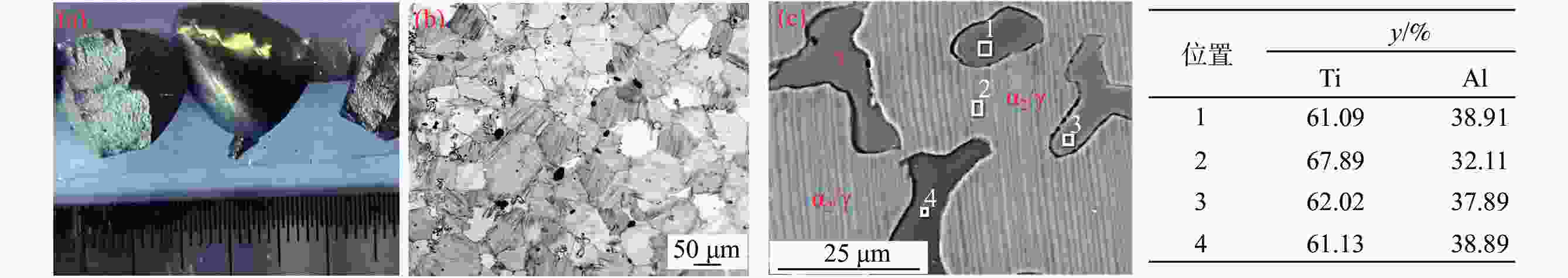
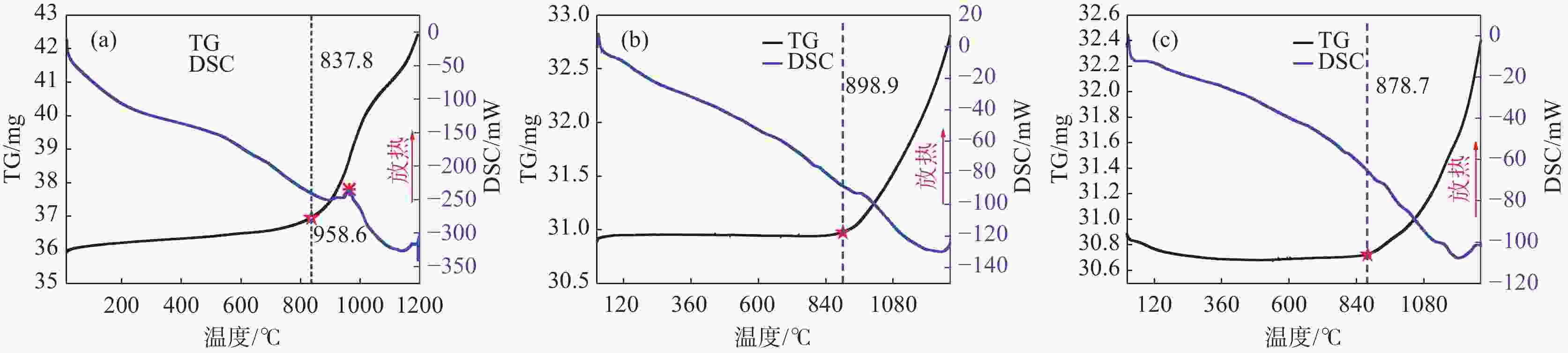
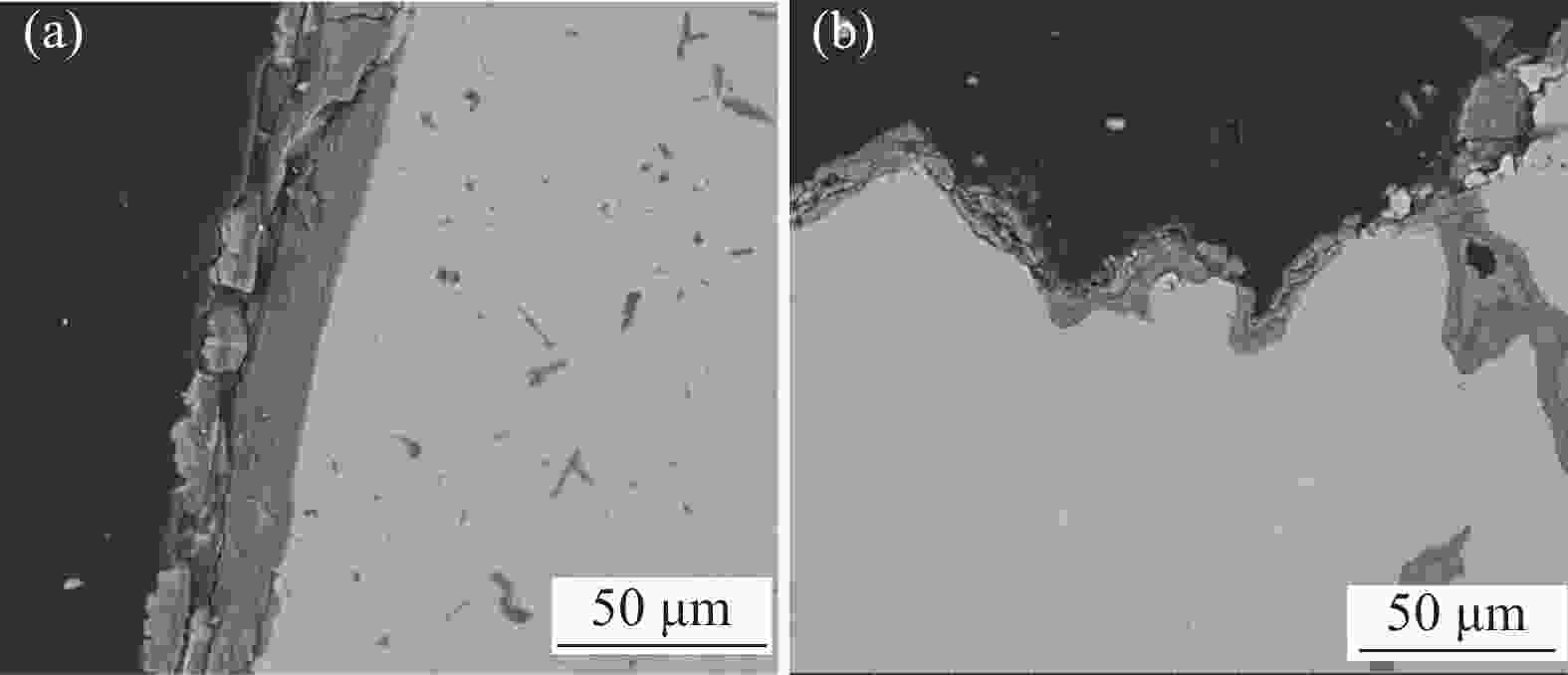
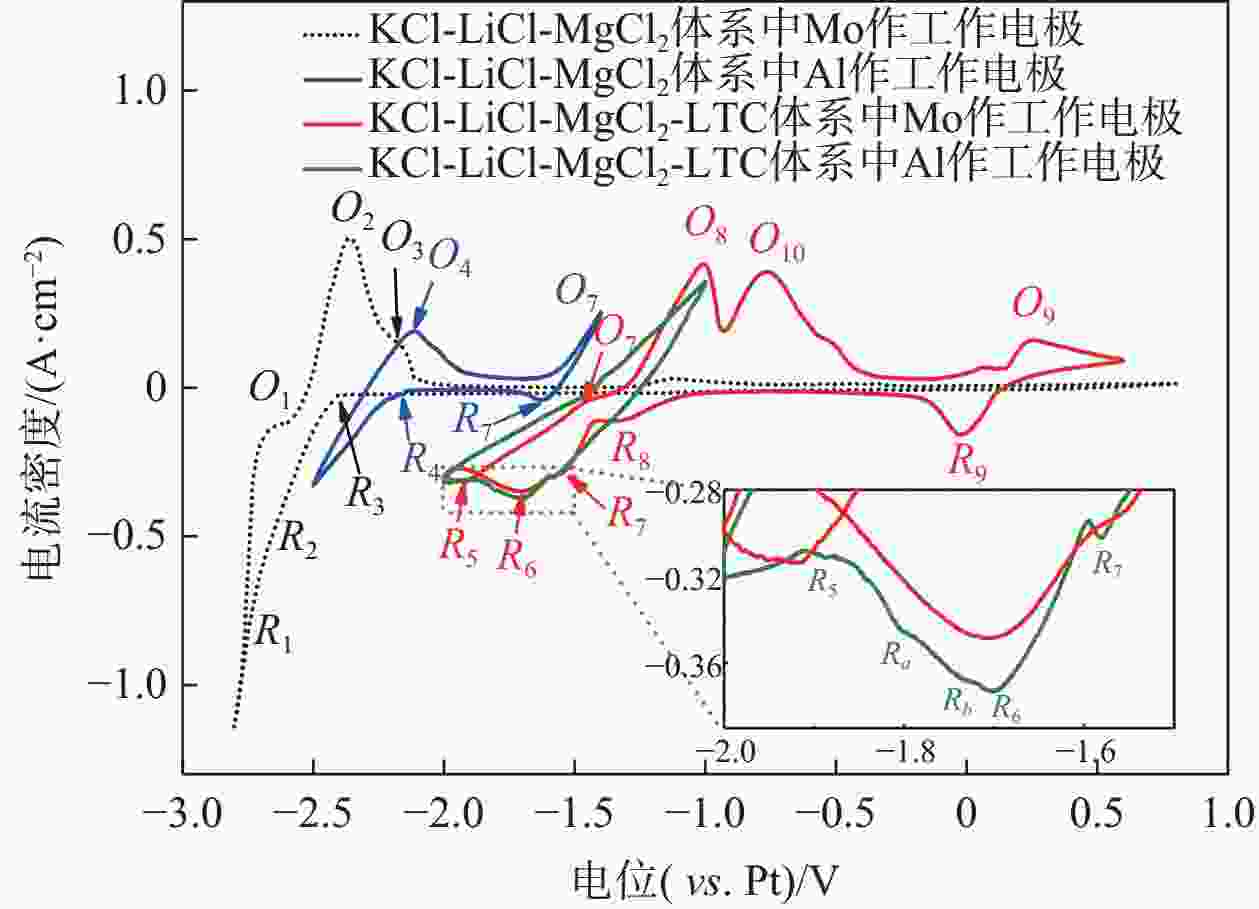
摘要: 针对低价氯化钛(LTC)浆料熔盐电解制备钛铝合金存在的电化学行为研究不系统、合金产品的高温抗氧化特性不明晰等问题,采用电化学工作站、示差热重分析仪等手段对LTC浆料在熔盐体系中电化学行为及合金产品的高温抗氧化特性进行研究。结果表明,LTC浆料在熔盐体系中可直接电化学还原制备Ti-Al合金,且为逐级还原的历程:Ti3+→Ti2+,Al3+→Al,Ti3+/Al3+→Ti-Al合金和Ti2+→Ti;随着熔盐体系中Ti3+离子浓度的增加,钛铝合金的组成变化规律为:Al/Al3Ti→Al3Ti2/Al5Ti2→AlTi/Al0.64Ti0.36→Al0.64Ti0.36/AlTi3→AlTi3→AlTi3/Ti→Ti,产品形貌变化规律为:粗枝晶→细枝晶→细球状结构→粗球状团聚体→细球状团聚体→细球多孔状→多孔状结构。钛铝合金中随着铝含量和产品致密性的增加,合金的抗高温氧化性能逐渐增强,Al0.64Ti0.36/AlTi3合金经高温熔炼后呈Al含量更高的α2/γ和γ组织,使其高温抗氧化性能优于商用Ti48Al2Cr2Nb。钛铝合金产品高温氧化历程为:Ti-Al合金→铝酸钛→金红石/氧化铝,并且形成的氧化物层可有效防止钛铝合金的进一步氧化。Abstract: Owing to the lack of systematic research on electrochemical behaviors and unclear high-temperature oxidation resistance characteristics of Ti-Al alloys prepared from low-valence titanium chlorides slurry by the electrochemical reduction in molten salt, the electrochemical workstation and differential thermal gravimetric analyzer were used to study the electrochemical behavior of LTC slurry in the molten salt system and the high-temperature oxidation resistance characteristics of alloy products. The results show that LTC slurry can be directly electrochemically reduced to Ti-Al alloys, following a step-by-step reduction process: Ti3+→Ti2+, Al3+→Al, Ti3+/Al3+→Ti-Al alloys, and Ti2+→Ti. As the concentration of Ti3+ ions in molten salts increases, the composition of Ti-Al alloys changes as follows: Al/Al3Ti→Al3Ti2/Al5Ti2→AlTi/Al0.64Ti0.36→Al0.64Ti0.36/AlTi3→AlTi3→AlTi3/Ti→Ti. The variation law of product morphology is as follows: coarse dendrite→fine dendrite→fine spherical structure→coarse spherical aggregate→fine spherical aggregate→fine spherical porous structure→porous structure. With the increase of aluminum content and product density in Ti-Al alloys, the high-temperature oxidation resistance gradually enhances. After high-temperature melting, Al0.64Ti0.36/AlTi3 alloy shows α2 and γ phase structures with a higher Al content, and its high-temperature oxidation resistance is superior to commercial Ti48Al2Cr2Nb. The high-temperature oxidation process of Ti-Al alloys is as follows: Ti-Al alloys→titanium aluminate→rutile/alumina, and the formed oxide layer can effectively prevent further oxidation.
-
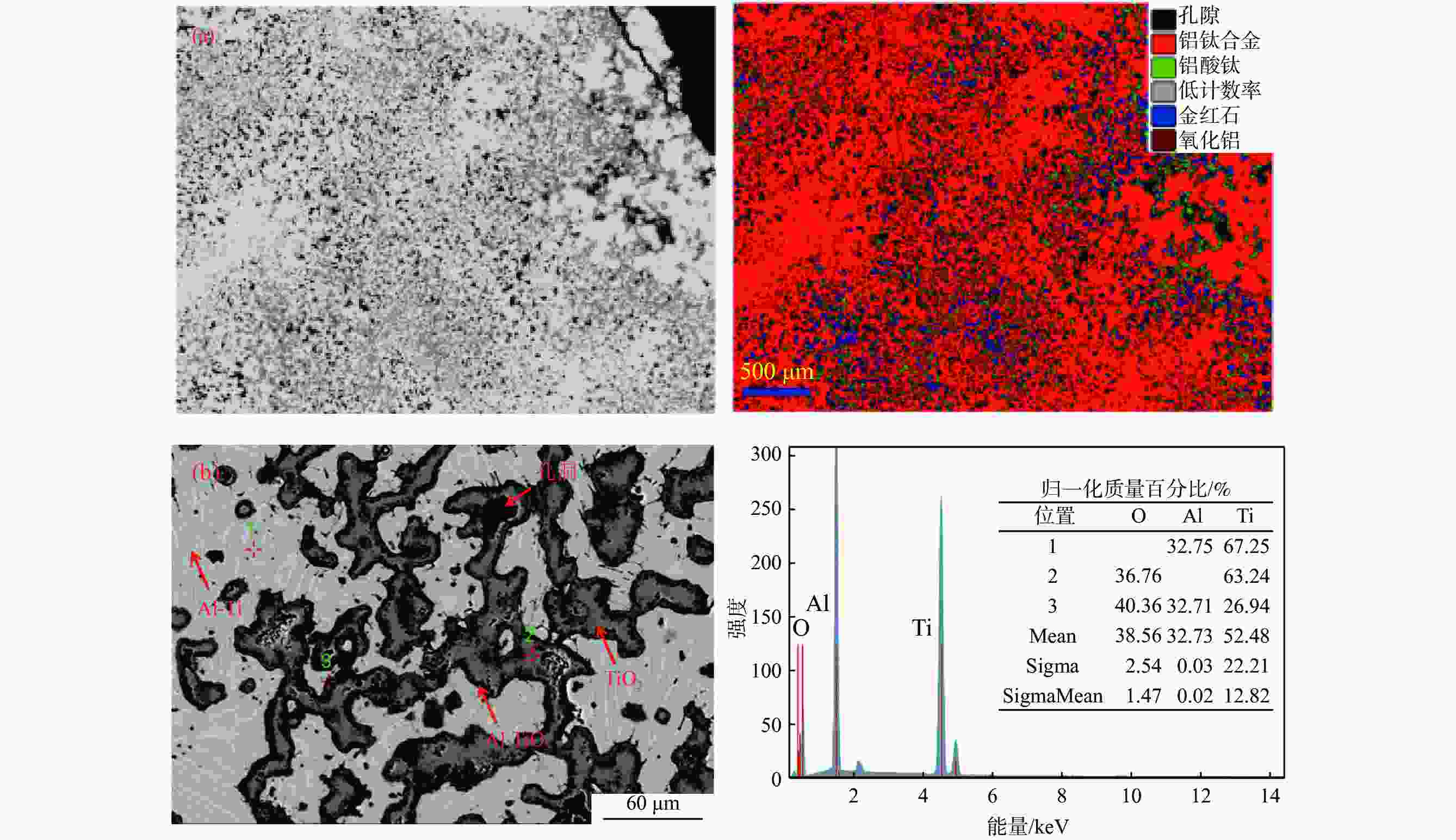
表 1 电解质中[Ti]/([Ti]+[Al])比例对阴极产物组成及形貌的影响
Table 1. The effect of molar ratios of [Ti]/([Ti]+[Al]) in electrolytes on the compositions and morphology of cathodic products
电解质[Ti]/([Ti]+[Al]) 阴极产物[Ti]/([Ti]+[Al]) 物相组成 形貌特征 0.15 0.182 Al、Al3Ti 粗大枝晶 0.38 0.394 Al3Ti2,Al5Ti2 细枝晶 0.50 0.485 AlTi、Al0.64Ti0.36 细球状结构 0.69 0.725 Al0.64Ti0.36、AlTi3 粗状况团聚体 0.77 0.764 AlTi3 细球状团聚体 0.83 0.880 AlTi3、Ti 细球多孔状 1.00 1.000 Ti 多孔状 表 2 钛铝合金锭高温氧化后重量变化情况
Table 2. Weight changes of Ti-Al alloy ingot after high-temperature oxidation
合金类别 氧化前/g 氧化后/g 增重率/% 钛铝合金锭 3.018 3.059 1.36 Ti48Al2Cr2Nb 1.222 1.229 0.57 -
[1] Wu L, Xia J, Cao H, et al. Improving the high-temperature oxidation resistance of TiAl alloy by anodizing in methanol/NaF solution[J]. Oxidation of Metals, 2018,90:617−631. doi: 10.1007/s11085-018-9858-1 [2] Wu X. Review of alloy and process development of TiAl alloys[J]. Intermetallics, 2006,14(10-11):1114−1122. doi: 10.1016/j.intermet.2005.10.019 [3] Chen Yuyong, Zhang Shuzhi, Kong Fantao, et al. Progress in β-solidifying γ-TiAl based alloys[J]. Chinese Journal of Rare Metals, 2012,36(1):154−160. (陈玉勇, 张树志, 孔凡涛, 等. 新型β-γTiAl合金的研究进展[J]. 稀有金属, 2012,36(1):154−160. doi: 10.3969/j.issn.0258-7076.2012.01.027 Chen Yuyong, Zhang Shuzhi, Kong Fantao, et al . Progress in β-solidifying γ-TiAl based alloys[J]. Chinese Journal of Rare Metals,2012 ,36 (1 ):154 −160 . doi: 10.3969/j.issn.0258-7076.2012.01.027[4] Zhang X, Li C, Wu M, et al. Atypical pathways for lamellar and twinning transformations in rapidly solidified TiAl alloy[J]. Acta Materialia, 2022,227:117718. doi: 10.1016/j.actamat.2022.117718 [5] Ouyang Hongwu, Liu Yong, He Yuehui, et al. Development and application of TiAl base alloy valve[J]. Materials Reports, 2003,17(4):8−10. (欧阳鸿武, 刘咏, 贺跃辉, 等. TiAl基合金排气阀的研制和应用前景[J]. 材料导报, 2003,17(4):8−10. Ouyang Hongwu, Liu Yong, He Yuehui, et al . Development and application of TiAl base alloy valve[J]. Materials Reports,2003 ,17 (4 ):8 −10 .[6] Yuan Naiqiang, Xu Yong, Xu Rongfu, et al. Research on the casting process of high Nb-TiAl alloy exhaust valve[J]. Foundry Technology, 2018,39(12):2728−2731. (袁乃强, 徐勇, 许荣福, 等. 高Nb-TiAl合金排气阀铸造成形工艺研究[J]. 铸造技术, 2018,39(12):2728−2731. Yuan Naiqiang, Xu Yong, Xu Rongfu, et al . Research on the casting process of high Nb-TiAl alloy exhaust valve[J]. Foundry Technology,2018 ,39 (12 ):2728 −2731 .[7] Yang Rui. Advances and challenges of TiAl base alloys[J]. Acta Metallurgica Sinica, 2015,51(2):129−147. (杨锐. 钛铝金属间化合物的进展与挑战[J]. 金属学报, 2015,51(2):129−147. doi: 10.11900/0412.1961.2014.00396 Yang Rui . Advances and challenges of TiAl base alloys[J]. Acta Metallurgica Sinica,2015 ,51 (2 ):129 −147 . doi: 10.11900/0412.1961.2014.00396[8] Wang Mengguang, Sun Jianke, Chen Zhiqiang. Current status on melting and casting process of gama TiAl based alloy[J]. Titanium Industry Progress, 2010(4):1−4. (王孟光, 孙建科, 陈志强. TiAl基合金的熔炼与铸造成形工艺研究现状[J]. 钛工业进展, 2010(4):1−4. Wang Mengguang, Sun Jianke, Chen Zhiqiang . Current status on melting and casting process of gama TiAl based alloy[J]. Titanium Industry Progress,2010 (4 ):1 −4 .[9] Yu Lanlan, Mao Xiaonan, Zhang Yingming, et al. Development of electron-beam cold hearth single melt process for titanium alloy ingots[J]. Titanium Industry Progress, 2009(2):14−18. (于兰兰, 毛小南, 张英明, 等. 电子束冷床炉单次熔炼钛合金铸锭研究进展[J]. 钛工业进展, 2009(2):14−18. doi: 10.3969/j.issn.1009-9964.2009.02.003 Yu Lanlan, Mao Xiaonan, Zhang Yingming, et al . Development of electron-beam cold hearth single melt process for titanium alloy ingots[J]. Titanium Industry Progress,2009 (2 ):14 −18 . doi: 10.3969/j.issn.1009-9964.2009.02.003[10] Narayana P L, Li C L, Kim S W, et al. High strength and ductility of electron beam melted β stabilized γ-TiAl alloy at 800 ℃[J]. Materials Science and Engineering, 2019,756(5):41−45. [11] Hu D, Dolganov A, Ma M, et al. Development of the Fray-Farthing-Chen Cambridge process: towards the sustainable production of titanium and its alloys[J]. JOM, 2018,70(2):129−137. doi: 10.1007/s11837-017-2664-4 [12] Yan B, Yan Y, Zhang M, et al. Electrochemical formation of titanium aluminum alloys from Ti2O3 in-situ chloridized by AlCl3 in chloride melts[J]. Electrochimical Acta, 2016,188:269−276. doi: 10.1016/j.electacta.2015.11.137 [13] Lahiri A, Das R. Spectroscopic studies of the ionic liquid during the electrodeposition of Al–Ti alloy in 1-ethyl-3-methylimidazolium chloride melt[J]. Materials Chemistry & Physics, 2012,132(1):34−38. [14] He Hualin, Qiu Kehui, Sun Zhaohui, et al. Heat balance calculation for preparation of vanadium removal slurry[J]. Chinese Journal of Rare Metals, 2016,40(2):61−65. (何华林, 邱克辉, 孙朝晖, 等. 除钒浆液制备过程的热平衡计算[J]. 稀有金属, 2016,40(2):61−65. He Hualin, Qiu Kehui, Sun Zhaohui, et al . Heat balance calculation for preparation of vanadium removal slurry[J]. Chinese Journal of Rare Metals,2016 ,40 (2 ):61 −65 .[15] Li Liang, Li Kaihua, Miao Qingdong, et al. Preparation and application of vanadium removing reagent in refining crude TiCl4[J]. Chinese Journal of Rare Metals, 2015,39(7):666−672. (李亮, 李开华, 苗庆东, 等. 四氯化钛精制除钒试剂的制备及应用研究[J]. 稀有金属, 2015,39(7):666−672. Li Liang, Li Kaihua, Miao Qingdong, et al . Preparation and application of vanadium removing reagent in refining crude TiCl4[J]. Chinese Journal of Rare Metals,2015 ,39 (7 ):666 −672 .[16] Miao Qingdong, Li Kaihua, He Anxi, et al. Preparation and application of TiCl3 slurry used in Al-powder vanadium removal of crude TiCl4[J]. Chinese Journal of Rare Metals, 2017(41):1369−1373. (苗庆东, 李开华, 何安西, 等. 粗四氯化钛铝粉除钒用TiCl3浆液制备及应用[J]. 稀有金属, 2017(41):1369−1373. Miao Qingdong, Li Kaihua, He Anxi, et al . Preparation and application of TiCl3 slurry used in Al-powder vanadium removal of crude TiCl4[J]. Chinese Journal of Rare Metals,2017 (41 ):1369 −1373 .[17] Zhu F X, Li L, Cheng X Z, et al. Direct electrochemical reduction of low titanium chlorides into titanium aluminide alloy powders from molten eutectic KCl–LiCl–MgCl2[J]. Electrochimical Acta, 2020,357:1−10. [18] Zhu F, Li K, Song W, et al. Composition and structure of Ti-Al alloy powders formed by electrochemical co-deposition in KCl-LiCl-MgCl2-TiCl3-AlCl3 molten salt[J]. Intermetallics, 2021,139:107341. doi: 10.1016/j.intermet.2021.107341 [19] Kim S, Matsunaga N, Kuroda K, et al. Effect of [Al(DMSO2)3]3+ concentration on Al electrodeposition from AlCl3/dimethylsulfone baths[J]. Journal of Electrochemical Science and Technology, 2018,9(1):69−77. doi: 10.33961/JECST.2018.9.1.69 [20] Zhu F, Li L, Song W, et al. Electrochemical synthesis of Ti-Al-V alloy by chlorination of Ti2O3 and V2O3 in AlCl3-containing molten chloride salt[J]. Journal Materials Research and Technology, 2021,13:1243−1253. doi: 10.1016/j.jmrt.2021.05.063 [21] Song J, Mukherjee A. Influence of F- on the electrochemical properties of titanium ions and Al-Ti alloy electrodeposition in molten AlCl3-NaCl[J]. RSC Advances, 2020,6:82049−82056. -




 下载:
下载: