Effect of TiO2 on viscosity and structure of high-temperature slag wool melts
-
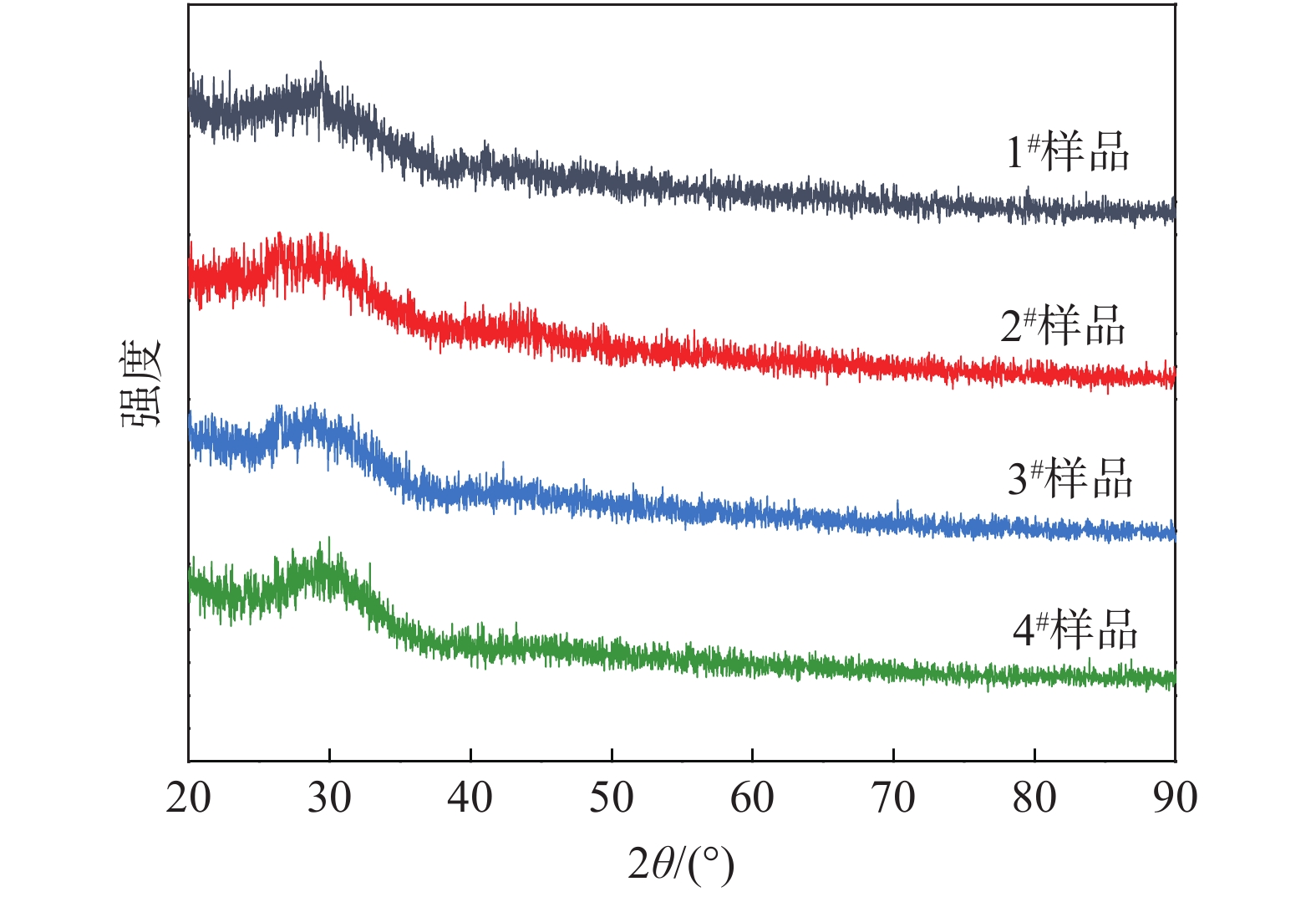
摘要: 为了研究TiO2含量的变化对矿渣棉高温熔体黏度的影响,以CaO–MgO–Al2O3–SiO2–TiO2五元渣系为研究对象,采用内柱体旋转法,系统研究了酸度系数为1.4时矿渣棉高温熔体的黏度变化规律,并结合拉曼光谱分析,探索了熔渣结构的变化特性。结果表明:当TiO2含量从1%增加至4%时,熔体黏度逐渐降低,黏流活化能由170.45 kJ/mol降至158.62 kJ/mol。当温度高于1 350 ℃时,样品黏度均低于1.5 Pa·s,流动性良好。同时,熔体内硅氧四面体结构中的Q0和Q1不断增加,而Q2和Q3逐渐降低,平均桥氧数由1.69降至0.95,熔体结构聚合度减小。Ti–O键不断增多,降低了熔体结构的稳定性。Abstract: In order to study the effect of TiO2 on the viscosity of high-temperature slag wool melts, the CaO–MgO–Al2O3–SiO2–TiO2 based slag system was used as the research object. And the viscosity variation of the slag wool melt with the acidity coefficient of 1.4 was systematically studied by using the cylinder rotation method, and the changes of slag structure were analyzed by Raman spectra. The results show that when the content of TiO2 increases from 1% to 4%, the viscosity of the melt gradually decreases, and the activation energy of viscous flow decreases from 170.45 kJ/mol to 158.62 kJ/mol. When the temperature is higher than 1 350 ℃, the viscosity of the samples is lower than 1.5 Pa·s, which means the melts have well fluidity. Meanwhile, Q0 and Q1 within the [SiO4]-tetrahedral structure gradually increase, while Q2 and Q3 gradually decrease. The average number of bridge oxygen decreases from 1.69 to 0.95, and the polymerization degree of the melt structure decreases. The increase of the Ti–O bond reduces the stability of the melt structure.
-
Key words:
- acidic Ti-bearing slag /
- slag wool /
- viscosity /
- activation energy /
- melt structure /
- Raman spectra
-
表 1 试验渣系组成方案
Table 1. Experimental compositions of slags
编号 物料添加量 /g 预熔渣化学成分 /% Slag SiO2 CaO Al2O3 MgO CaO SiO2 Al2O3 MgO TiO2 Mk 0 100 38.89 31.11 14.19 8.74 5.35 1# 17.20 41.40 26.34 8.56 6.50 33.26 46.66 11.23 7.93 0.92 1.4 2# 37.38 34.44 17.75 5.70 4.73 32.74 46.38 10.89 8.01 1.98 1.4 3# 56.07 28.00 9.79 3.04 3.10 32.29 45.74 10.94 8.07 2.96 1.4 4# 74.77 21.55 1.83 0.39 1.47 31.82 44.91 11.08 8.12 4.07 1.4 表 2 TiO2含量对熔体黏流活化能的影响
Table 2. Effect of TiO2 on the viscous activation energy of melts
w(TiO2)/% 回归方程 拟合度(R2) 活化能Eη/(kJ•mol−1) 1 y=20 501.66x−12.36 0.994 4 170.45 2 y=19 705.38x−11.97 0.998 0 163.83 3 y=19 580.75x−12.04 0.996 3 162.79 4 y=19 079.13x−11.77 0.995 4 158.62 表 3 硅氧四面体中Qn的面积分数以及平均桥氧数计算结果
Table 3. The area fraction of Qn in silicon-oxygen tetrahedron and the calculation results of mean bridging oxygen number
序号 Q0 /% Q1/% Q2/% Q3 /% 平均桥氧数 1 16.54 23.85 33.13 26.48 1.69 2 20.90 26.44 27.78 24.88 1.56 3 29.80 19.18 35.01 16.01 1.37 4 40.52 32.29 19.22 7.97 0.95 -
[1] Liu Baoyao, Zhang Xiaobing. Feasibility of producing basalt wool by blast furnace molten slag[J]. Multipurpose Utilization of Mineral Resources, 2006,(1):44−47. (刘保瑶, 张小兵. 熔融高炉渣制造玄武岩棉的可行性分析[J]. 矿产综合利用, 2006,(1):44−47. doi: 10.3969/j.issn.1000-6532.2006.01.012 [2] Huo Jixiang, Huang Junjie. Protection measures for No. 2 blast furnace in Shougang Jingtang[J]. Ironmaking, 2013,32(3):14−16. (霍吉祥, 黄俊杰. 首钢京唐2号高炉护炉措施[J]. 炼铁, 2013,32(3):14−16. [3] Gao Xuesheng, Han Xiupeng. Protection of No. 8 blast furnace in shougang Changgang[J]. Ironmaking, 2013,32(2):50−53. (高雪生, 韩秀鹏. 首钢长钢8号高炉护炉实践[J]. 炼铁, 2013,32(2):50−53. [4] Yan Zhiming, Lv Xuewei, He Wenchao, et al. Effect of TiO2 on the liquid zone and apparent viscosity of SiO2-CaO-8%MgO-14%Al2O3 system[J]. ISIJ International, 2017,57(1):31−36. doi: 10.2355/isijinternational.ISIJINT-2016-420 [5] Hyunsik Park, Jun-Young Park, Gi Hyun Kim, et al. Effect of TiO2 on the viscosity and slag structure in blast furnace type slags[J]. Steel Research International, 2012,83(2):150−156. doi: 10.1002/srin.201100249 [6] Gao Yanhong, Bian Lingtao, Liang Zhongyu. Influence of B2O3 and TiO2 on viscosity of titanium-bearing blast furnace slag[J]. Steel Research International, 2015,86:386−390. doi: 10.1002/srin.201400039 [7] Rohindra D R, Lata R A, Coll R K. A simple experiment to determine the activation energy of the viscous flow of polymer solutions using a glass capillary viscometer[J]. European Journal of Physics, 2012,33(5):1457. doi: 10.1088/0143-0807/33/5/1457 [8] Shi Zhe, Xiong Hongjin. Analysis on the viscosity of six different compound smelting reduction slag[J]. Journal of Chongqing University, 2014,37(5):37−45. (施哲, 熊洪进. 六元熔融还原渣黏度的分析[J]. 重庆大学学报, 2014,37(5):37−45. doi: 10.11835/j.issn.1000-582X.2014.05.006 [9] Zhang Kai, Zhang Zuotai, Liu Lili, et al. Investigation of the viscosity and structural properties of CaO-SiO2-TiO2 slags[J]. Metallurgical and Materials Transactions B, 2014,45(4):1389−1397. doi: 10.1007/s11663-014-0053-8 [10] Jiao Kexin, Zhang Jianliang, Wang Zhiyu, et al. Effect of TiO2 and FeO on the viscosity and structure of blast furnace primary slags[J]. Steel Research International, 2017,88(5):1600296. doi: 10.1002/srin.201600296 [11] Mysen B O , Virgo D , Scarfe C M. Relations between the anionic structure and viscosity of silicate melts-a Raman spectroscopic study[J]. American Mineralogist, 1980,65(7−8):690−710. [12] SunYongqi, Zhang Zuotai, Liu Lili, et al. FTIR, Raman and NMR investigation of CaO-SiO2-P2O5 and CaO-SiO2-TiO2-P2O5 glasses[J]. Journal of Non-Crystalline Solids, 2015,420:26−33. doi: 10.1016/j.jnoncrysol.2015.04.017 [13] Deng Leibo, Zhang Xuefeng, Zhang Mingxing, et al. Effect of CaF2 on viscosity, structure and properties of CaO-Al2O3-MgO-SiO2 slag glass ceramics[J]. Journal of Non-Crystalline Solids, 2018,500:310−316. doi: 10.1016/j.jnoncrysol.2018.08.018 [14] Xing Xiangdong, Pang Zhuogang, Mo Chan, et al. Effect of MgO and BaO on viscosity and structure of blast furnace slag[J]. Journal of Non-Crystalline Solids, 2020,530:119801. doi: 10.1016/j.jnoncrysol.2019.119801 -





 下载:
下载: