Study on preparation of vanadium trioxide by hydrogen reduction
-
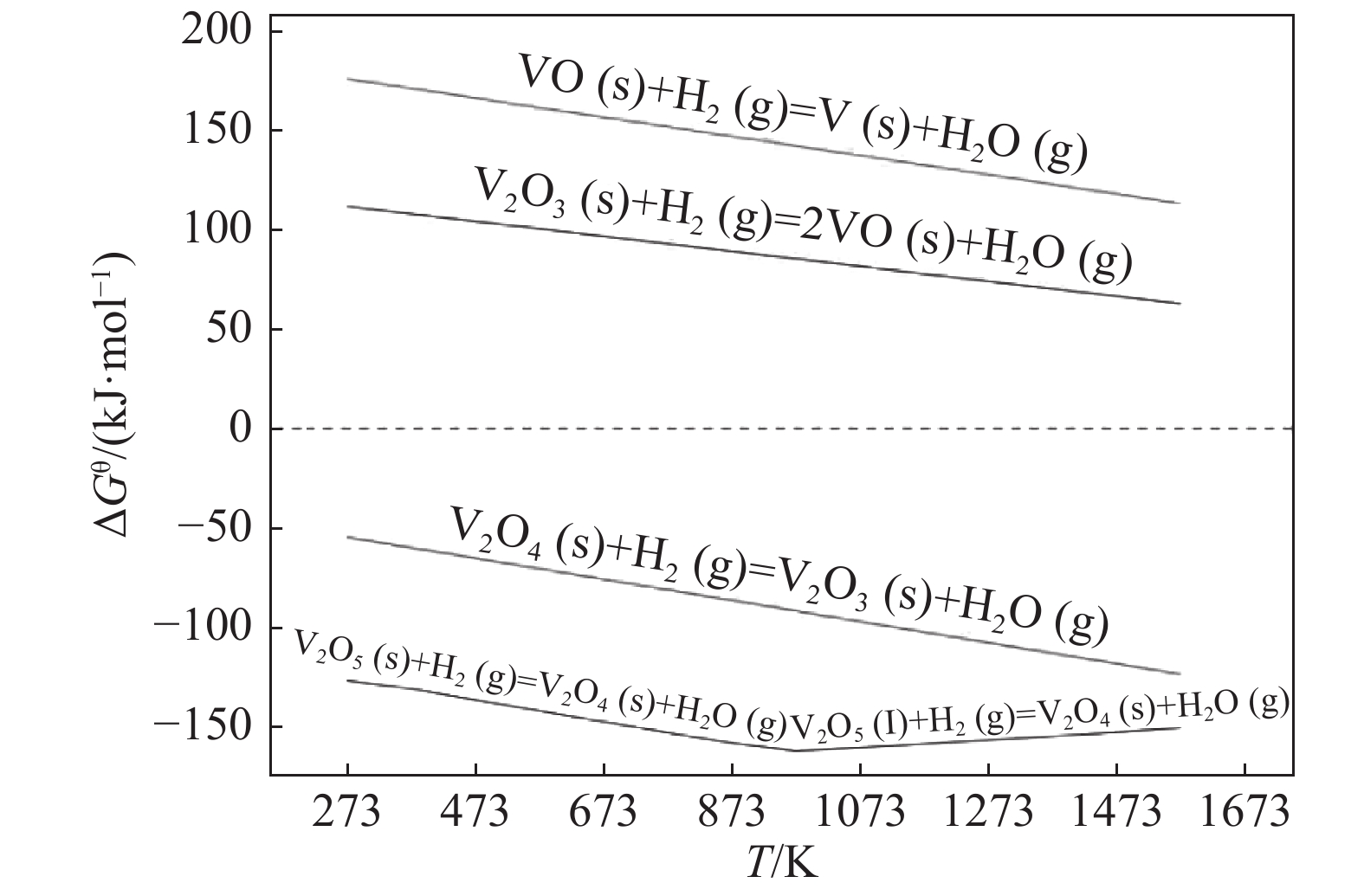
摘要: 对氢气还原制备V2O3进行了系统研究,通过V-H-O体系中钒还原氧化热力学分析确定不同条件下钒的稳定存在状态,并分析了反应温度和时间对还原脱氧的影响,获得了氢气还原制备三氧化二钒的最佳工艺参数,在最佳条件下可获得钒含量为67.81%的V2O3产品,同时采用X衍射分析揭示了V2O5还原脱氧过程的物相变化规律:氢气还原V2O5是逐级进行的,还原过程中形成中间产物V3O16和V3O7;随着还原程度的不断增加,聚合物逐渐被解离形成V2O3和VO2,最后VO2被全部还原为单一稳定相V2O3。Abstract: In this paper, the preparation of V2O3 by hydrogen reduction was systematically studied. The stable state of vanadium under different conditions was determined by thermodynamic analysis of vanadium redox reactions in V-H-O system. The influence of reaction temperature and time on deoxidation was analyzed, and the optimum reduction conditions were obtained. Under the optimum conditions, the V2O3 product with a vanadium content of 67.81% can be obtained. At the same time, the phase changes of V2O5 during the reduction process were revealed via X-ray diffraction. It shows a stepwise reduction process of V2O5, with the intermediate products of V3O16 and V3O7 formed during the reduction. As the reduction proceeds, the intermediates can be gradually decomposed into V2O3 and VO2. Finally, VO2 is completely reduced into V2O3.
-
表 1 五氧化二钒的主要化学成分
Table 1. The main chemical compositions of V2O5
% TV Fe Si K Na S 55.95 0.017 0.080 0.007 0.016 0.013 -
[1] Yu Bin, Sun Zhaohui, Chen Haijun, et al. Preparation and influence factors of FeV50 alloy based on gradient distribution of Al addition[J]. Chinese Journal of Rare Metals, 2017,41(11):1279−1284. (余彬, 孙朝晖, 陈海军, 等. 基于梯度配铝的FeV50 合金制备及其影响因素[J]. 稀有金属, 2017,41(11):1279−1284. [2] Yang Yangjun, Sun Zhaohui, Tang Hongguo, et al. Test study on FeV50 smelting with vanadium trioxide in electrosilicothermal process[J]. Iron Steel Vanadium Titanium, 2003,24(2):20−23. (杨仰军, 孙朝晖, 唐洪国, 等. 用三氧化二钒电硅热法冶炼FeV50试验研究[J]. 钢铁钒钛, 2003,24(2):20−23. [3] (杨勇. 高效低成本钒氮合金制备关键工艺技术研究[D]. 北京: 钢铁研究总院, 2018.)Yang Yong. Study on key technology of high efficiency and low cost vanadium nitrogen alloy preparation[D]. Beijing: Central Iron & Steel Research Institude, 2018. [4] Pei Guishang, Xiang Junyi, Zhong Dapeng, et al. A clean process of preparing VO as LIBs anode materials via the reduction of V2O3 powder in a H2 atmosphere: Thermodynamic assessment, isothermal kinetic analysis, and electrochemistry performance evaluation[J]. Journal of Alloys and Compounds, 2020,845:1−9. [5] Zhou Jing, Xiao Hansheng,Weng Wei, et al. Interfacial confinement of Ni-V2O3 in molten salts for enhanced electrocatalytic hydrogen evolution[J]. Journal of Energy Chemistry, 2020,50:280−285. doi: 10.1016/j.jechem.2020.03.048 [6] Wu Yuedong, Zhang Guohua, Xu Rui, et al. Fabrication of pure V2O3 powders by reducing V2O5 powders with CO-CO2 mixed gases[J]. Ceramics International, 2019,45:2117−2123. doi: 10.1016/j.ceramint.2018.10.117 [7] Wang Fengkang, Xu Baoqiang, Wan Heli, et al. Preparation of vanadium powders by calcium vapor reduction of V2O3 under vacuum[J]. Vacuum, 2020,173:1−4. [8] Zhang Fan. Research on preparation of vanadium trioxide by means of fluidization[J]. Iron Steel Vanadium Titanium, 2008,24(2):27−30. (张帆. 流态化制取三氧化二钒研究[J]. 钢铁钒钛, 2008,24(2):27−30. doi: 10.7513/j.issn.1004-7638.2008.02.007 [9] Zheng Shaobo. Basic research on hydrogen metallurgy and new ironmaking idea-process[J]. China Metallurgy, 2012,22(7):1−6. (郑少波. 氢冶金基础研究及新工艺探索[J]. 中国冶金, 2012,22(7):1−6. -





 下载:
下载: