Study on the effect and mechanism of Al3+ during the calcination of metatitanic acid
-
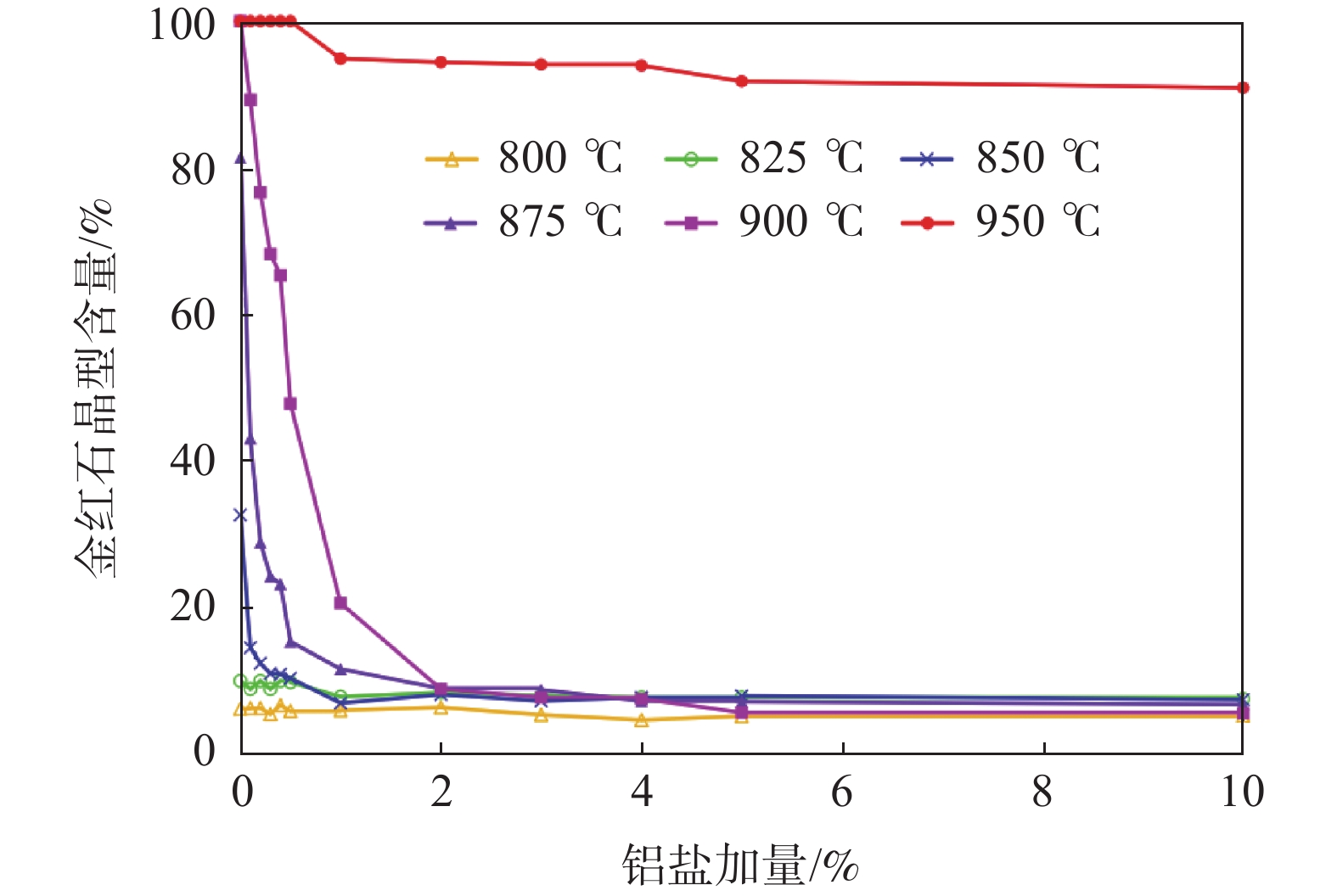
摘要: 硫酸法钛白生产工艺中,偏钛酸经不同盐处理和高温煅烧后获得适宜粒径的金红石二氧化钛是制备颜料钛白的基础,铝系盐处理是目前普遍采用的盐处理体系之一,但目前铝盐在煅烧过程的作用机制尚不明确。以硫酸法钛白生产中间物料二洗偏钛酸为原料,仅以硫酸铝作为盐处理剂,在马弗炉里升温至800~950 ℃条件下煅烧。利用XRD、SEM、HRTEM等分析手段研究了Al3+在煅烧过程中对TiO2晶型转变和粒子生长的影响。结果表明,不同铝盐加量以及煅烧温度对TiO2晶型转变、粒子生长以及Al3+的存在状态具有显著影响。铝盐加量较低时,Al3+以取代模式掺入TiO2晶格中,置换Ti4+,使晶胞体积变小,晶体内部键长变短,不利于原子重排和断键过程,导致晶型转变率降低。随着铝盐加量的增加,过量的Al3+在TiO2表面以Al2O3的形式存在,同时相同煅烧温度下得到的锐钛型或金红石型TiO2粒子粒径减小。Abstract: In the production of titanium dioxide by sulfate process, obtaining rutile titanium dioxide with suitable particle size through different salt treatments and high-temperature calcination of metatitanic acid is the basis for preparing titanium pigments. Aluminum salt treatment is currently one of the commonly used salt treatment systems, but the mechanism of aluminum salt in the calcination process is still unclear. In this study, the intermediate material in the production of titanium dioxide by sulfate process, metatitanic acid after second washing, was used as raw material, and only aluminum sulfate was used as salt treatment agent. The material was calcined in a muffle furnace at a temperature of 800-950 ℃. The effects of Al3+on the crystal transformation and particle growth of TiO2 during the calcination process were studied using XRD, SEM, HRTEM, and other analytical methods. The results show that different amounts of aluminum salts and calcination temperatures have significant effects on the crystal transformation, particle growth, and existence form of Al3+ in TiO2. When the amount of aluminum salt is low, Al3+ is doped into the TiO2 lattice in a substitution mode, displacing Ti4+, reducing the crystal cell volume and shortening the internal bond length of the crystal, which is not conducive to the atomic rearrangement and bond breaking processes, leading to a decrease in the crystalline transformation rate. And with the increase of the amount of aluminum salt added, excessive Al3+ exists as Al2O3 on the TiO2 surface. At the same time, with the increase of the amount of aluminum salt added, the particle size of anatase or rutile TiO2 obtained at the same calcination temperature decreases.
-
Key words:
- titanium dioxide /
- metatitanic acid /
- Al ion doping /
- crystal transformation
-
图 2 (a) P-A-825和A-y-825 (y=0.1,0.2,0.3,0.4,0.5,1,2,3,4,5,10);(b)锐钛(101)晶面;(c) P-A-950和A-y-950 (y=0.1,0.2,0.3,0.4,0.5,1,2,3,4,5,10);(d)金红石(110)晶面的XRD谱图
Figure 2. XRD patterns of catalysts (a) P-A-825 and A-y-825 (y=0.1,0.2,0.3,0.4,0.5,1,2,3,4,5,10), (b) (101) face of anatase, (c) P-A-950 and A-y-950 (y=0.1,0.2,0.3,0.4,0.5,1,2,3,4,5,10) and (d) (110) face of rutile
表 1 TiO2晶体结构精修结果统计
Table 1. The data of TiO2 crystal structure refinement results
样品名称 晶胞参数 晶胞体积 /nm3 a/nm b/nm c/nm P-A-950 0.45929 0.45929 0.29586 6.2410 A-0.5-950 0.45928 0.45928 0.29586 6.2409 A-1-950 0.45927 0.45927 0.29585 6.2406 -
[1] Luo Wusheng, Yu Shengfei. Design of metatitanic acid calcining system based on thermal characteristics of titanium dioxide[J]. Inorganic Chemicals Industry, 2014,46(1):61−64. (罗武生, 喻胜飞. 基于二氧化钛热工特性的偏钛酸煅烧系统设计[J]. 无机盐工业, 2014,46(1):61−64.Luo Wusheng, Yu Shengfei. Design of metatitanic acid calcining system based on thermal characteristics of titanium dioxide[J]. Inorganic Chemicals Industry, 2014, 46(1): 61-64. [2] Wang Z, Chen K, Zhu J, et al. Formation mechanism of rutile in sulfate process[J]//IOP Conference Series. Materials Science and Engineering, 2019, 562: 012002. [3] 唐振宁. 钛白粉的生产与环境治理[M]. 北京: 化学工业出版社, 2000.Tang Zhenning. Production and environmental treatment of titanium dioxide[M]. Beijing: Chemical Industry Press, 2000. [4] Yang Qing. Effect of Al salt on morphology and pigment properties on titanium dioxide after calcination[J]. Chemical Enterprise Management, 2017,(33):85−86. (杨青. Al盐对钛白粉窑下物形貌和颜料性能的影响[J]. 化工管理, 2017,(33):85−86.Yang Qing. Effect of Al salt on morphology and pigment properties on titanium dioxide after calcination[J]. Chemical Enterprise Management, 2017(33): 85-86. [5] Gesenhues U, Rentschler T. Crystal growth and defect structure of Al3+-doped rutile[J]. Journal of Solid State Chemistry, 1999,143(2):210−218. doi: 10.1006/jssc.1998.8088 [6] Wang Zinan, Chen Kui, Zhu Jiawen, et al. Effects of crystal seeds and salt dopants on phase transformation of TiO2 in calcination process[J]. Inorganic Chemicals Industry, 2020,52(3):45−50. (王子楠, 陈葵, 朱家文, 等. 晶种和盐处理剂对煅烧过程中二氧化钛晶型转变的影响[J]. 无机盐工业, 2020,52(3):45−50.Wang Zinan, Chen Kui, Zhu Jiawen, et al. Effects of crystal seeds and salt dopants on phase transformation of TiO2 in calcination process[J]. Inorganic Chemicals Industry, 2020, 52(3): 45-50. [7] Zhang W, Zhu Z, Cheng C Y. Literature review of titanium metallurgical processes[J]. Hydrometallurgy, 2011,108:177−188. doi: 10.1016/j.hydromet.2011.04.005 [8] 倪月琴. 偏钛酸晶型转化的研究[D]. 天津: 天津大学, 2006.Ni Yueqin. Study on phase transformation of metatitanic acid[D] . Tianjin: Tianjin University, 2006. [9] De L S, Aguilar T, Sánchez-Coronilla A, et al. Electronic and structural properties of highly aluminum ion doped TiO2 nanoparticles: A combined experimental and theoretical study[J]. Chem Phys Chem, 2014,15(11):2267−2280. doi: 10.1002/cphc.201402071 [10] Lee J E, Oh S, Park D. Synthesis of nano-sized Al doped TiO2 powders using thermal plasma[J]. Thin Solid Films, 2004,457(1):230−234. doi: 10.1016/j.tsf.2003.12.027 [11] Lee H, Lee D Y, Lee M, et al. Preparation of Al-TiO2 nanotubes and their photocatalytic activities[J]. Journal of Electroceramics, 2019,42(3-4):124−128. doi: 10.1007/s10832-018-0159-5 [12] Ishigaki T, Nakada Y, Tarutani N, et al. Enhanced visible-light photocatalytic activity of anatase-rutile mixed-phase nano-size powder given by high-temperature heat treatment[J]. Royal Society Open Science, 2020,7(1):191539. doi: 10.1098/rsos.191539 [13] Murashkina A A, Rudakova A V, Ryabchuk V K, et al. Influence of the dopant concentration on the photoelectrochemical behavior of Al-doped TiO2[J]. Journal of Physical Chemistry C, 2018,122(14):7975−7981. doi: 10.1021/acs.jpcc.7b12840 -





 下载:
下载: