Study on the effect of the particle size of hydrolysis seeds prepared by industrial titanyl sulfate solution
-
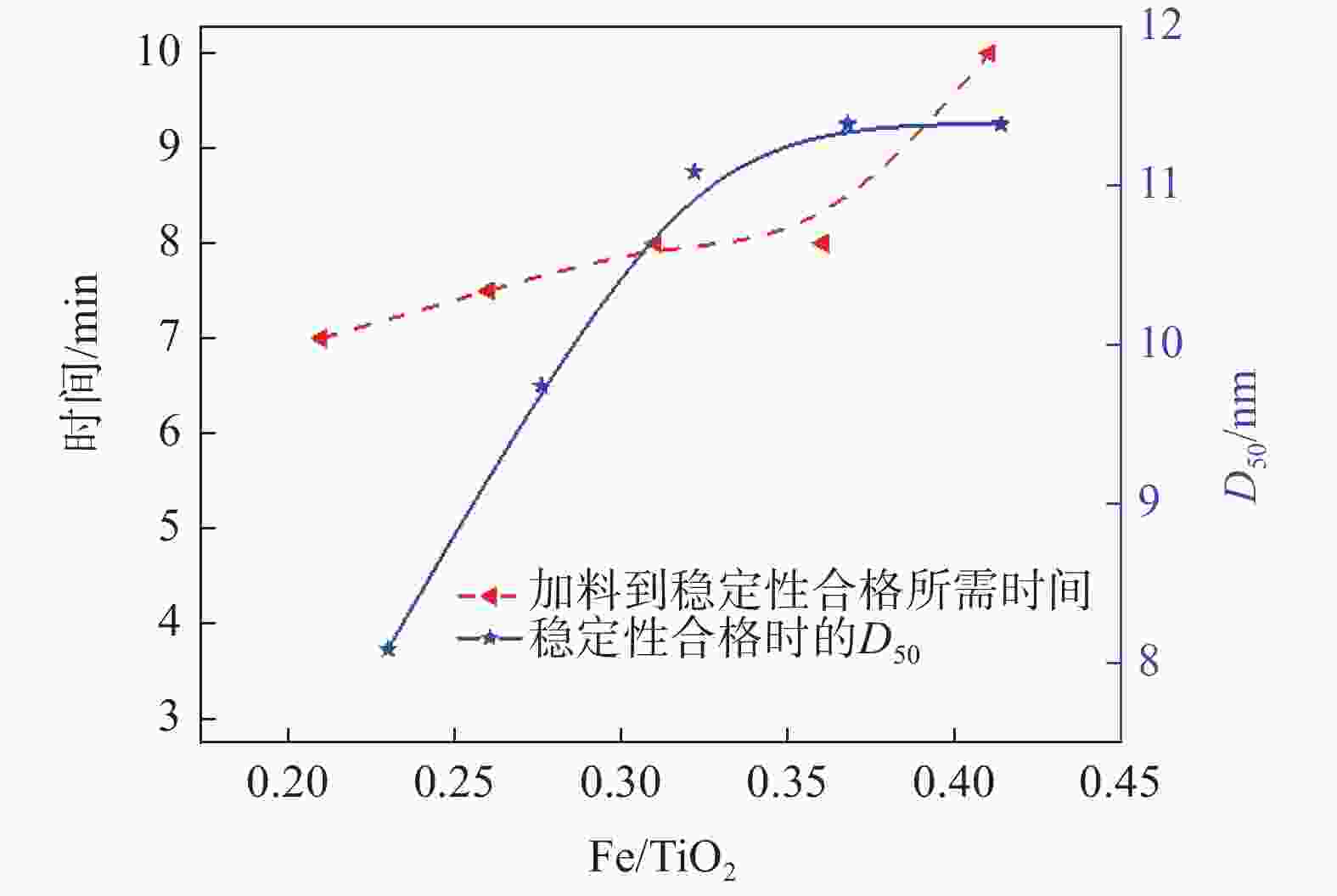
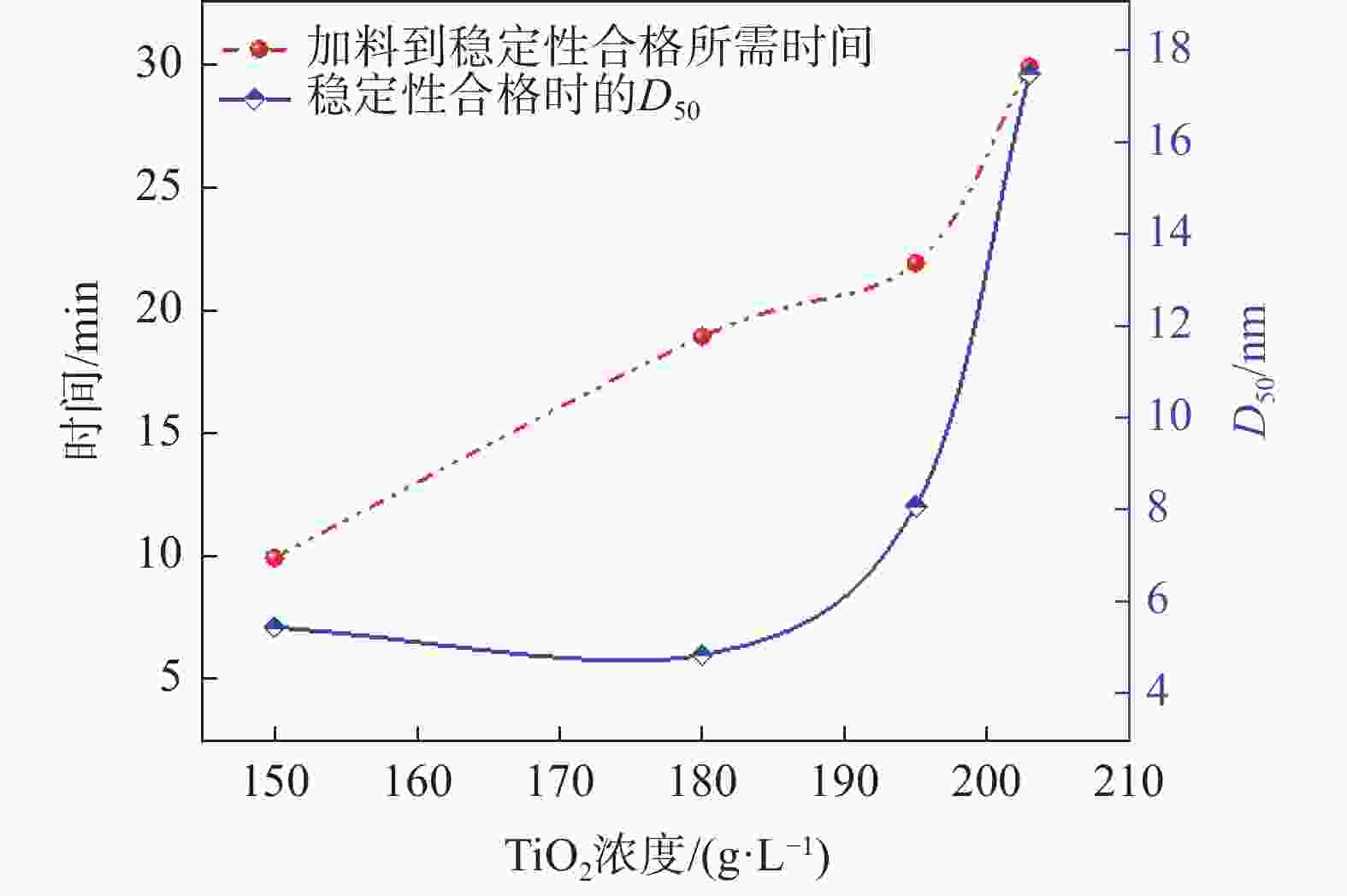
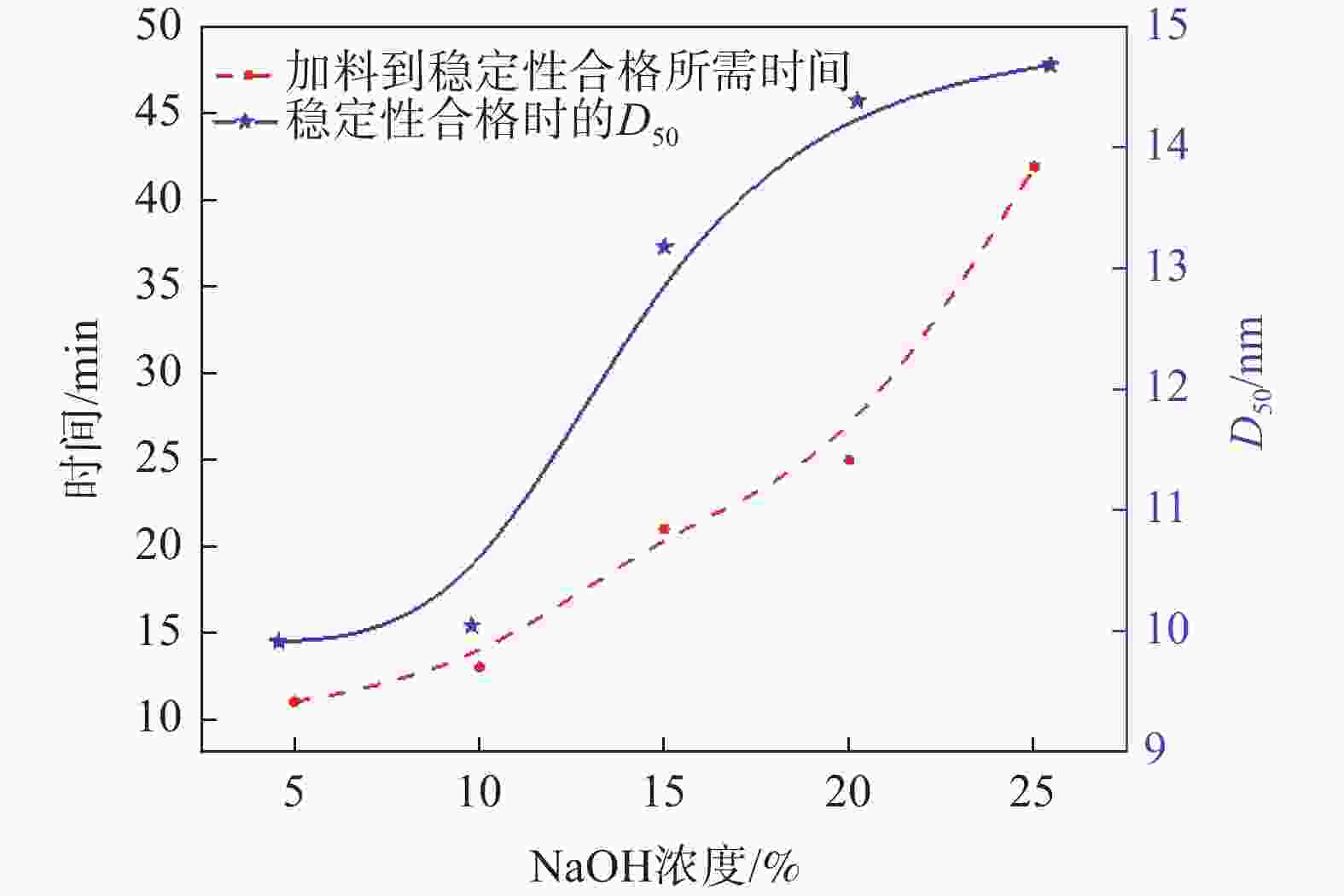
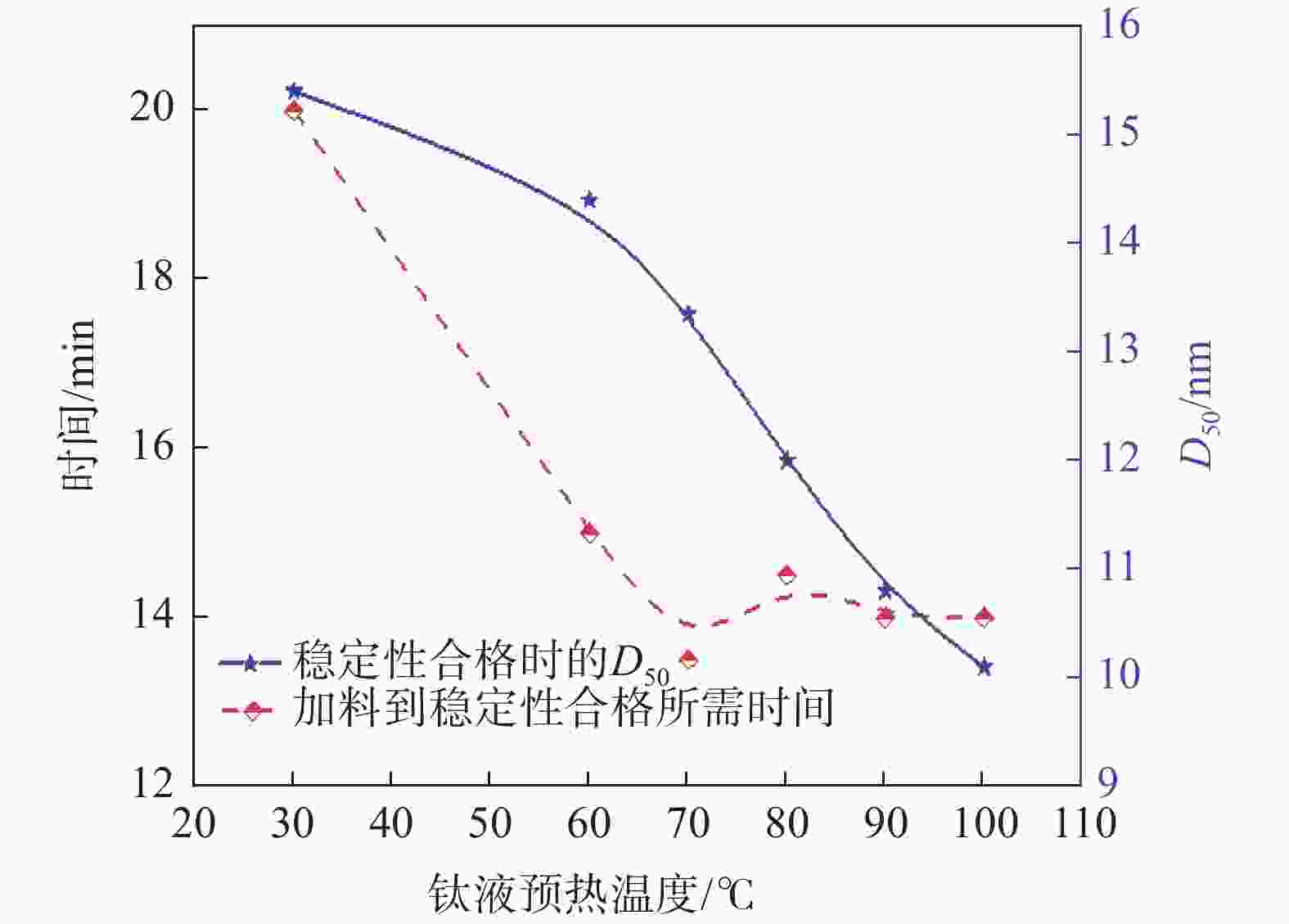
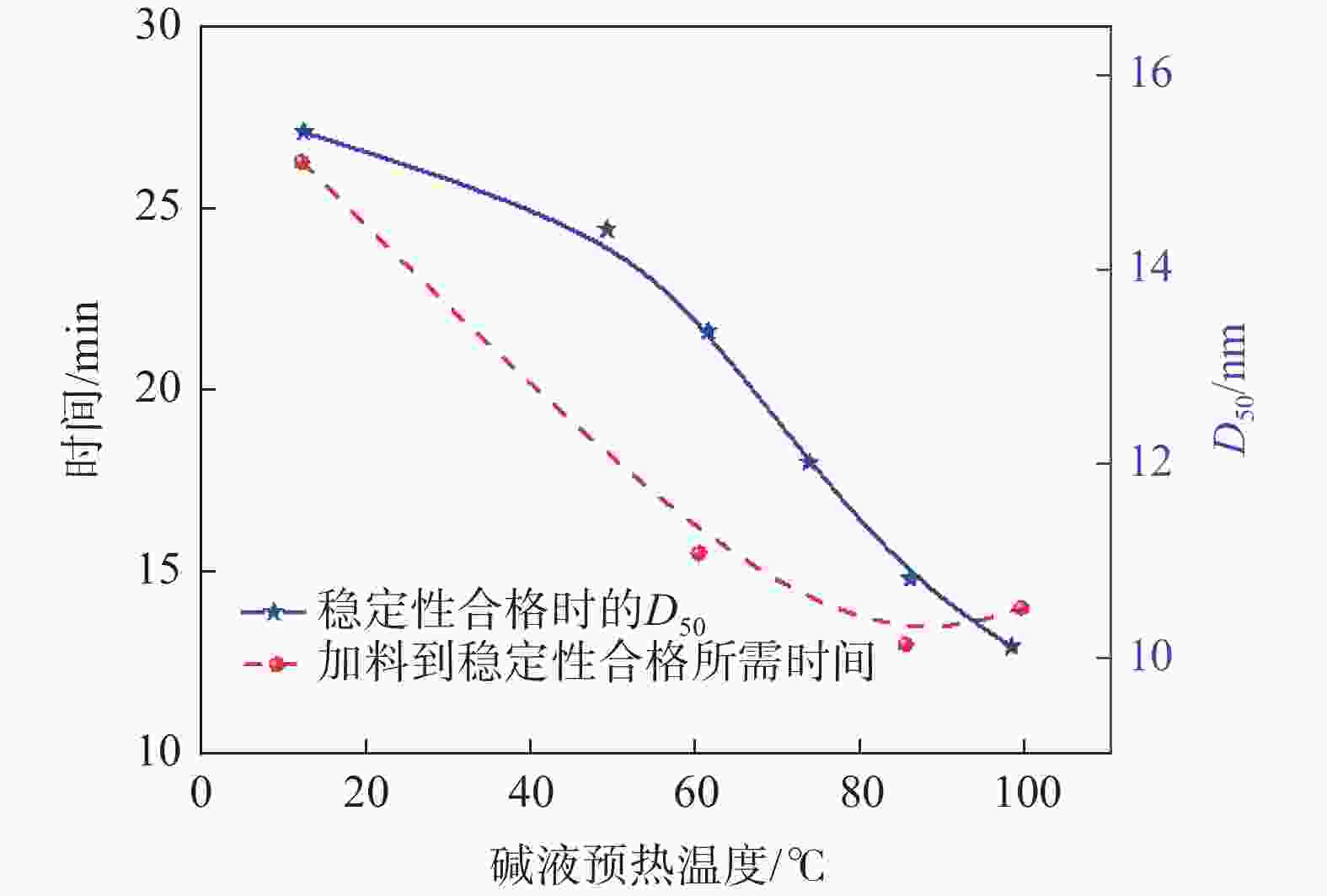
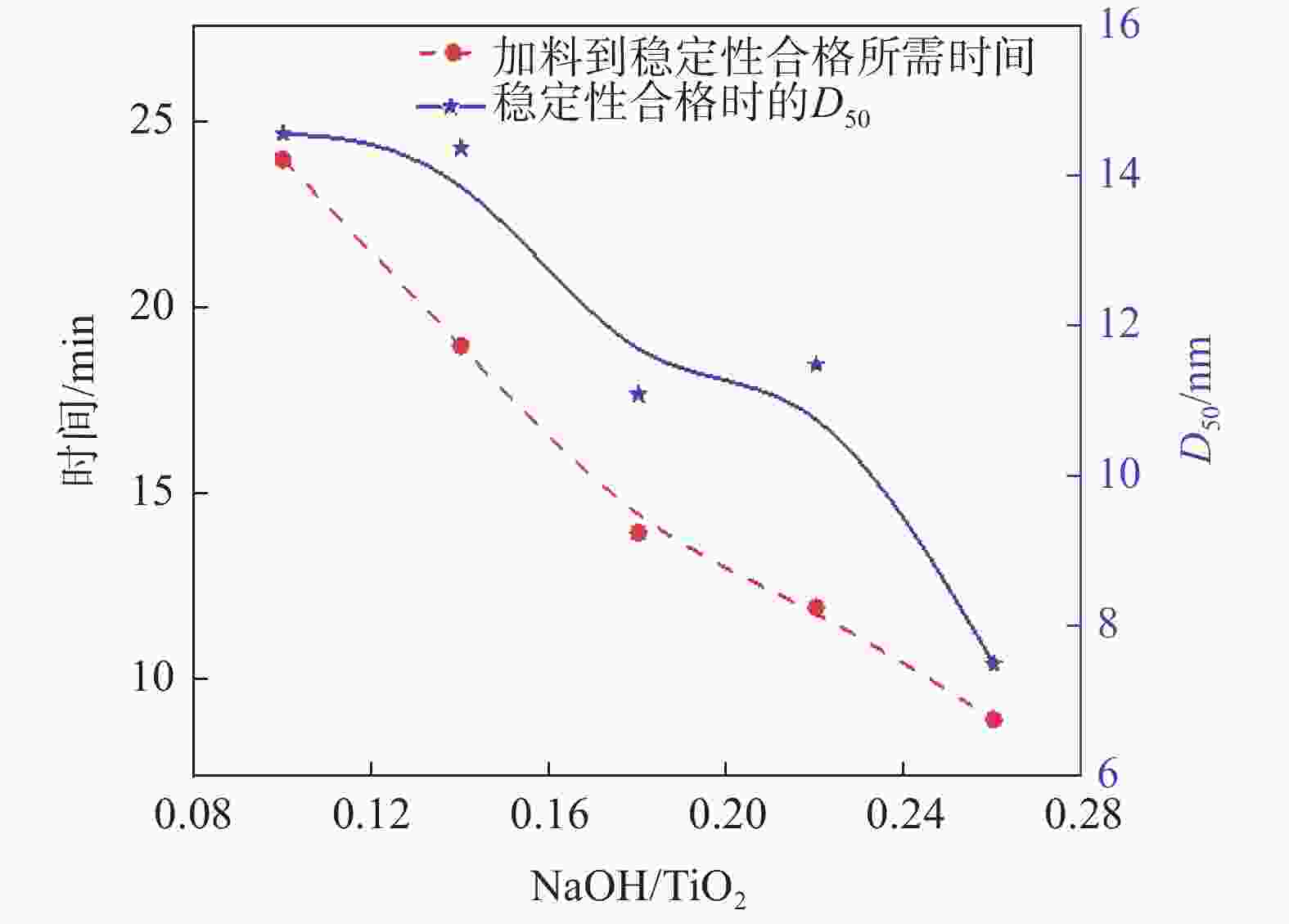
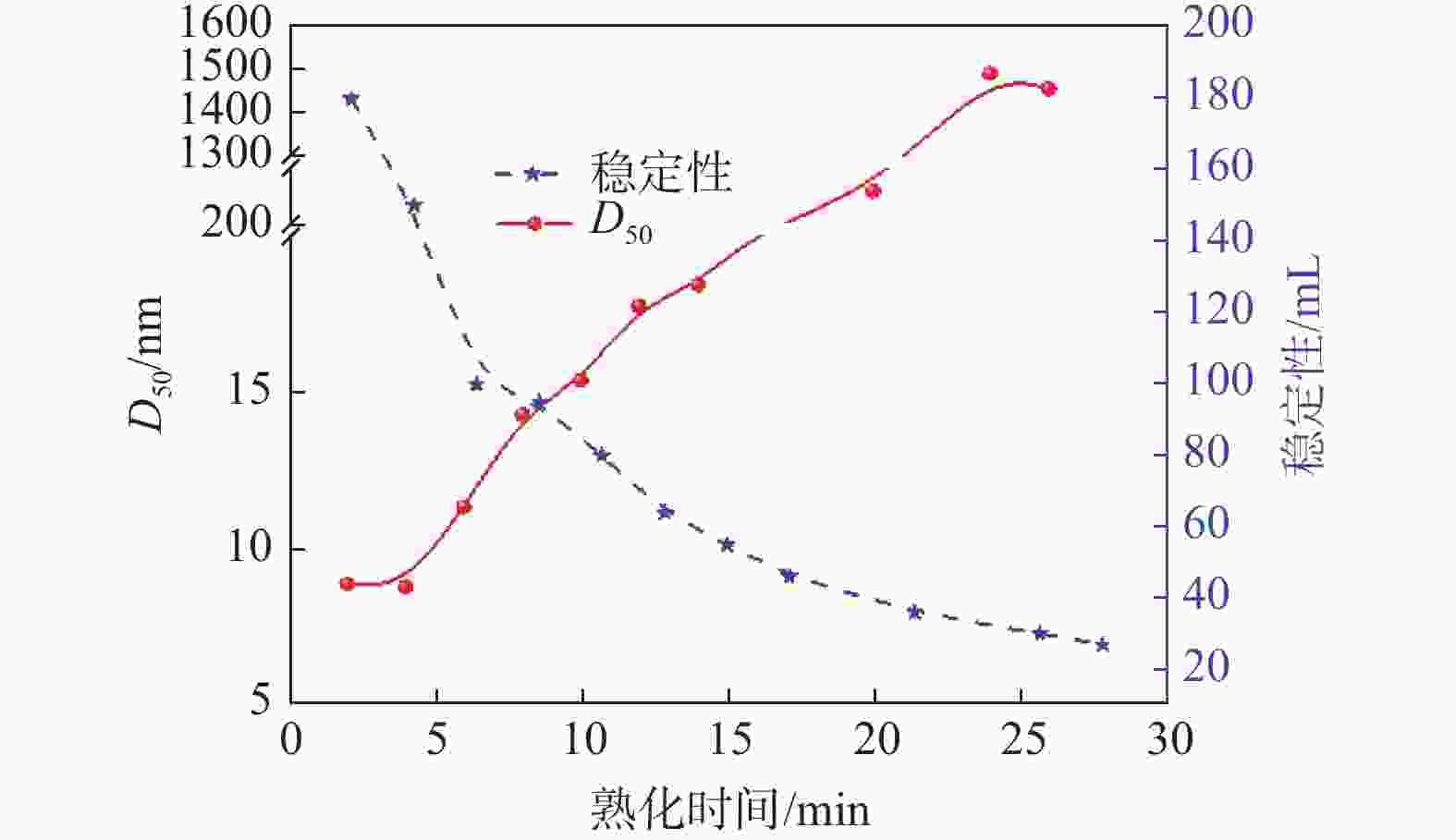
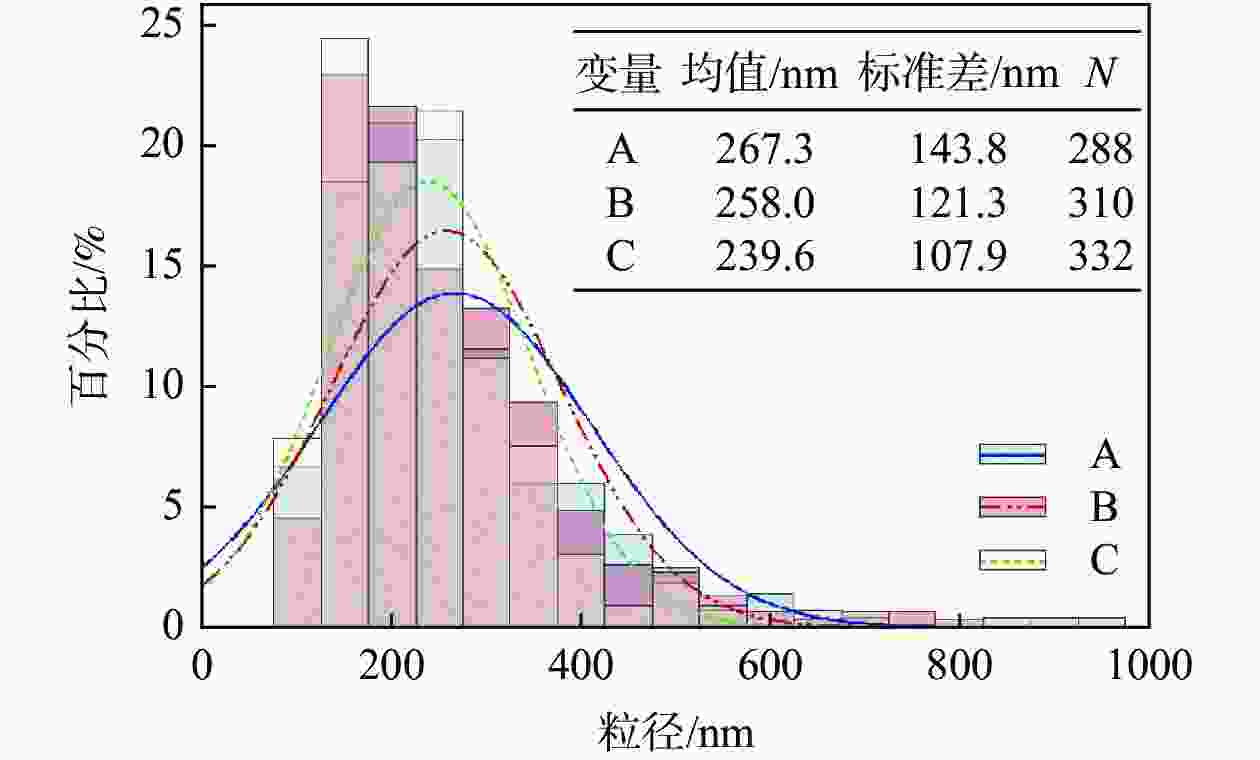
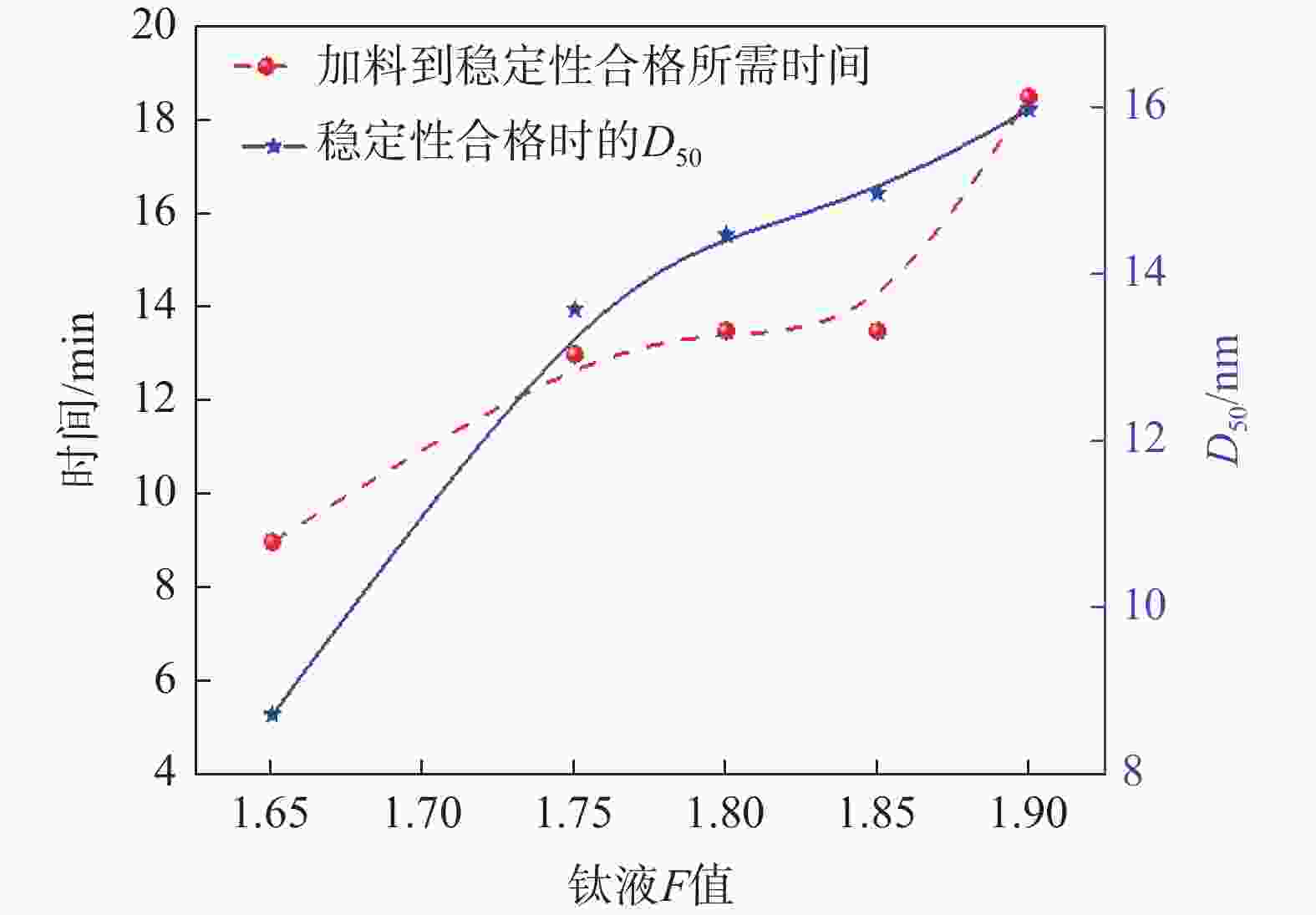
摘要: 以工业钛液为原料,采用目前硫酸法钛白企业常用的氢氧化钠溶液中和法制备水解晶种工艺,改变关键原料指标和工艺参数,考察晶种粒径和晶种稳定性的变化规律。然后将不同粒径的晶种用于水解,研究了晶种粒径对偏钛酸粒径及固定盐处理煅烧后所得金红石钛白初品粒径和消色力的影响。结果表明,随着钛液TiO2浓度、F值、铁钛比以及碱液浓度的升高,相同稳定性下晶种粒径变大;随着晶种制备碱钛比、钛液预热温度、碱液预热温度的增加,相同稳定性下晶种粒径减小;随着熟化时间的延长,晶种稳定性降低,晶种粒径变大。随着晶种粒径的变大,水解所得偏钛酸D50和径距逐渐变小,偏钛酸晶粒尺寸略增;对应金红石钛白初品的SEM平均粒径和标准差逐渐减小,同时其Tcs、Scx均增加。Abstract: With industrial titanyl sulfate solution as raw material, the hydrolysis seeds were prepared by sodium hydroxide solution neutralization method, which is commonly used in titanium pigments production by sulfuric process. And under different raw material indexes and process parameters, the changes of the seeds particle size stability were also investigated. Then the seeds with different particle sizes were used for hydrolysis, and the correlation between particle size of seeds and that of metatitanic acid was studied. Furthermore, under the condition of fixed salt treatment, the influence on the particle size and reducing power of the subsequent rutile titanium white was studied. The results show that with the increase of TiO2 concentration, F value, Fe/TiO2 of titanyl sulfate solution and NaOH solution concentration, the particle size of seeds increases under the same stability. With the increase of the ratio of NaOH/TiO2, the preheating temperature of titanyl sulfate solution and NaOH solution, the particle size of seeds decreases under the same stability. With the increase of aging time, the stability of seeds decreases and the particle size of seeds increases. With the increase of seeds particle size, D50 and span of metatitanic acid obtained by hydrolysis gradually decreases, and the grain size of metatitanic acid slightly increases. The SEM average particle size and standard deviation of the corresponding rutile titanium white sample gradually decreases, while the Tcs and Scx of the sample increases.
-
Key words:
- titanium white /
- hydrolysis /
- seeds /
- particle size /
- metatitanic acid
-
表 1 晶种粒径和对应的水解偏钛酸粒度分布
Table 1. Particle size of seeds and corresponding particle size distribution of metatitanic acid
编号 晶种粒
径
/nm偏钛酸粒径 偏钛酸晶粒
尺寸
/nmD10/μm D50/μm D90/μm 径距 A 7.73 0.882 2.48 5.27 1.77 6.0 B 11.8 0.897 2.38 4.69 1.59 6.1 C 15.5 0.888 2.26 4.26 1.49 6.3 表 2 不同晶种粒径对应的金红石初品的颜料性能
Table 2. Pigment properties of rutile TiO2 corresponding to different particle size of seeds
编号 R/% Scx Tcs Jasn Ton A 99.02 1.28 1723 94.67 −8.15 B 99.14 1.22 1735 94.66 −8.09 C 99.02 1.53 1747 94.71 −8.03 -
[1] Piccolo L, Paolinelli A, Pellizzon T. Process for the hydrolysis of titanium sulphate solutions: US, 4014977[P]. 1977-03-29. [2] Tian C, Huang S, Yang Y. Anatase TiO2 white pigment production from unenriched industrial titanyl sulfate solution via short sulfate process[J]. Dyes and Pigments, 2013,96(2):609−613. doi: 10.1016/j.dyepig.2012.09.016 [3] Santacesatia E. Kinetics of titanium dioxide precipitation by thermal hydrolysis[J]. Journal of Colloid and Interface Science, 1986,111(1):45−53. [4] Duncan J F, Richards R G. Solution equilibriums, kinetics and mechanism[J]. N. Z J. Sci., 1976,19(2):179−183. [5] Hao L, Wei H. On-line investigation of anatase precipitation from titanyl sulphate solution[J]. Chemical Engineering Research and Design, 2010,88:1264−1271. doi: 10.1016/j.cherd.2010.01.008 [6] 范兵. 硫酸法钛白自生晶种水解工艺条件优化的研究[D]. 郑州: 郑州大学, 2014.Fan Bing. Optimism of process conditions on self-generating seeded in the sulphate process[D]. Zhengzhou: Zhengzhou University, 2014. [7] 郝琳. 二氧化钛水解过程的系统研究及优化[D]. 天津: 天津大学, 2006.Hao Lin. Experimental investigation and optimization for titanium dioxide hydrolysis process[D]. Tianjin: Tianjin University, 2006. [8] Sekhar Sathyamoorthy, Moggridge Geoff D, Hounslow Michael J. Controlling particle size during anatase precipitation[J]. AIChE Journal, 2001,47:2012−2024. doi: 10.1002/aic.690470912 [9] Tang Siyang, Zhang Yaowen, Yuan Shaojun, et al. Microwave-assisted seed preparation for producing easily phase-transformed anatase to rutile[J]. RSC Adv., 2017,7:45607−45614. doi: 10.1039/C7RA07385B -




 下载:
下载: