Effect of alkali leaching and sludge strengthening on phosphorus removal of steel slag ceramics
-
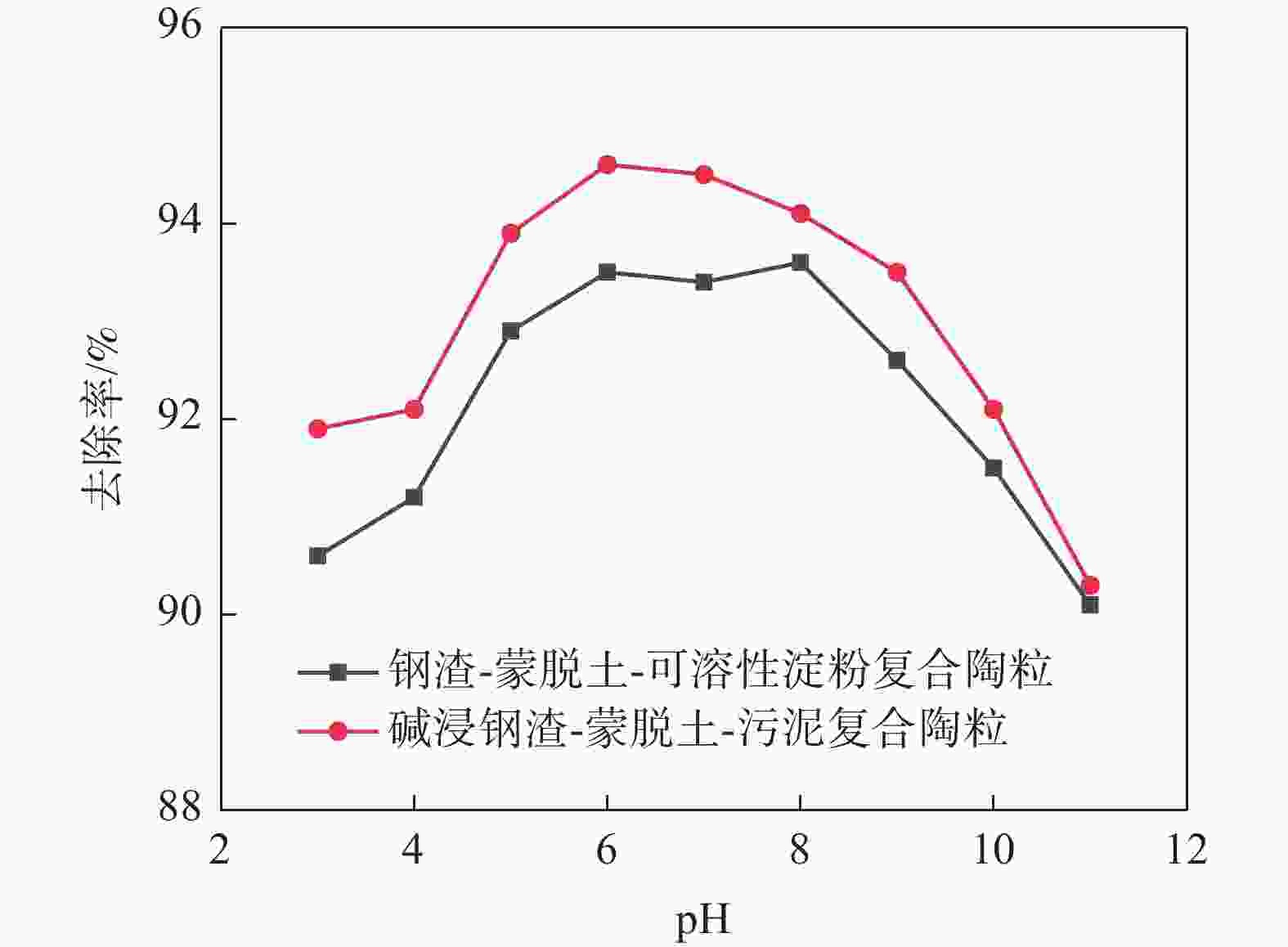
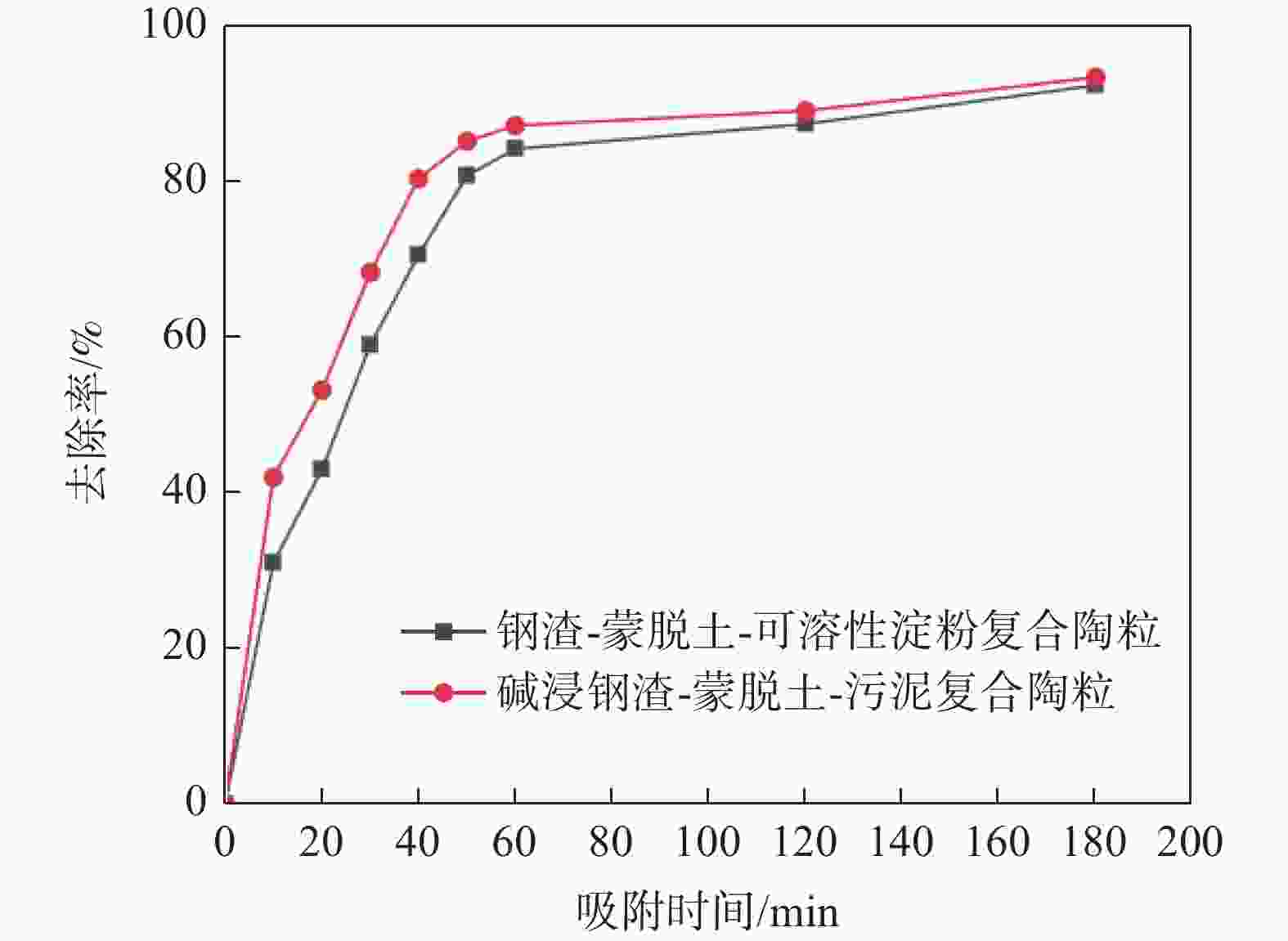
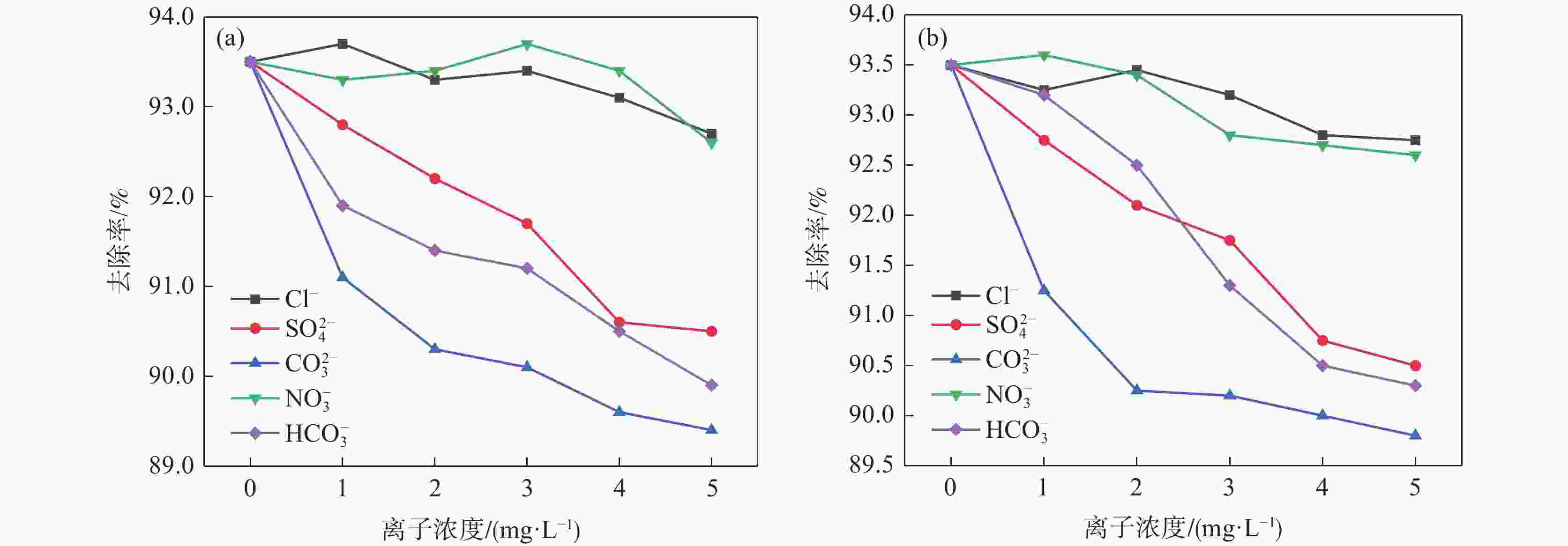
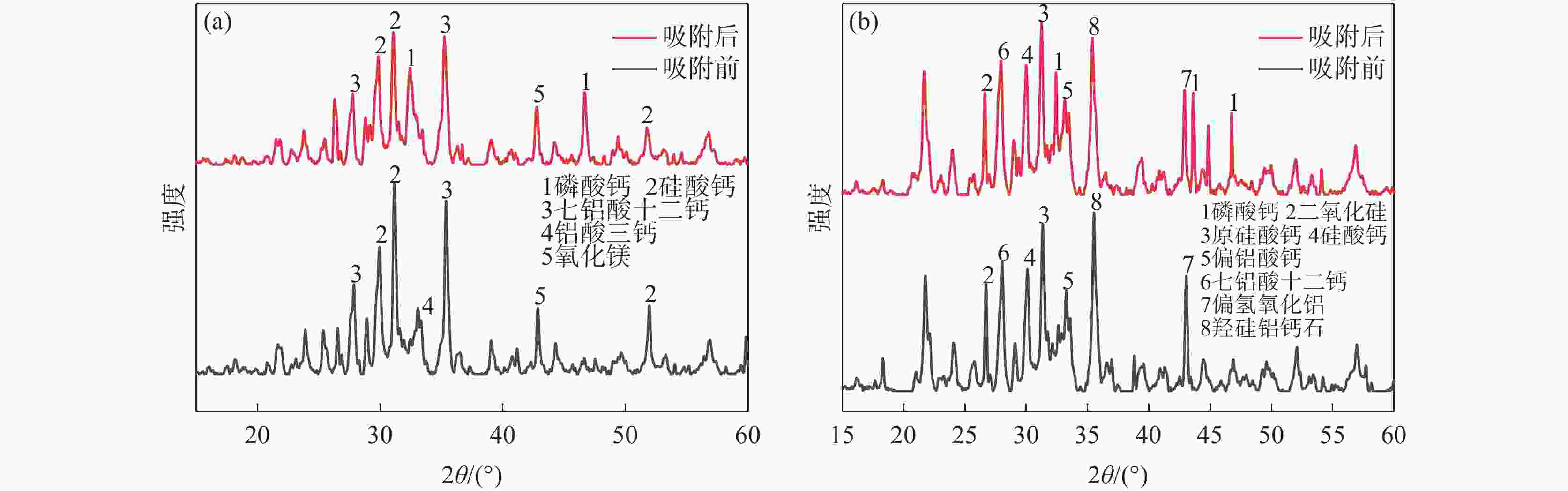
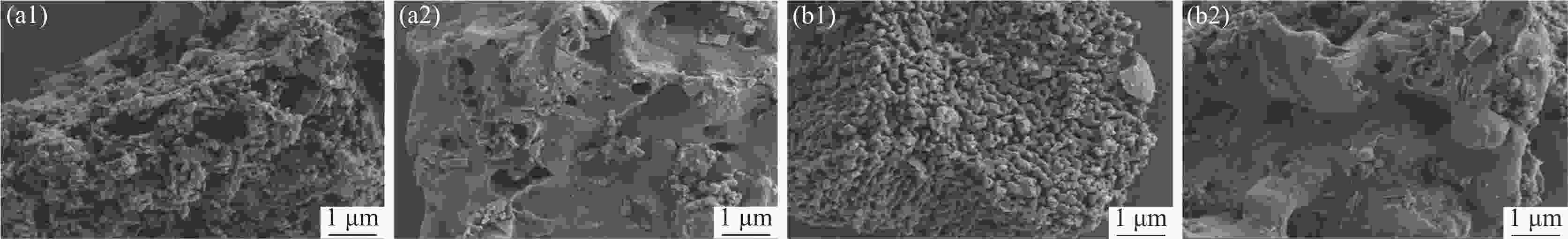
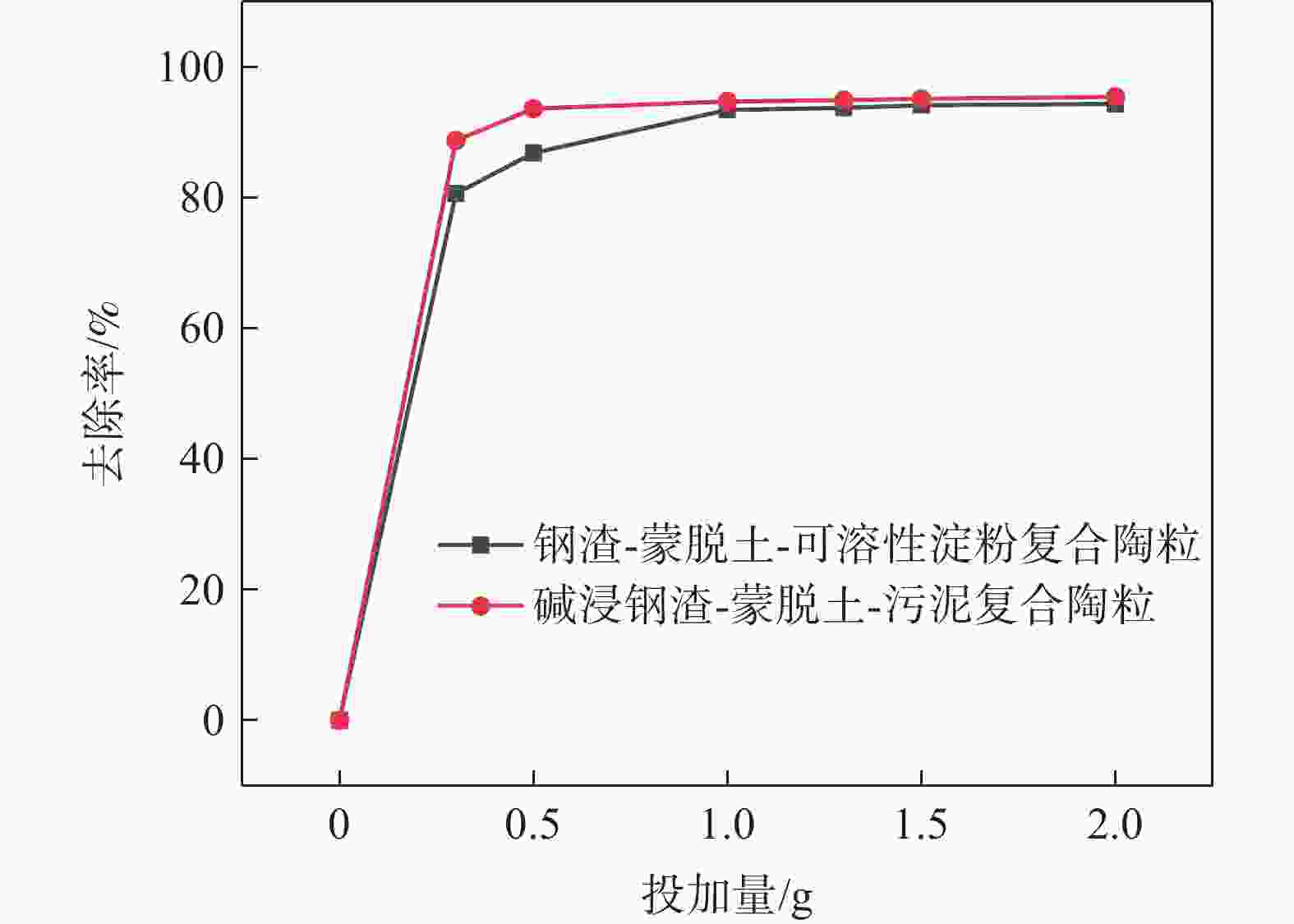
摘要: 为提高钢渣除磷效果,采用碱浸预处理,并添加污泥强化制备碱浸钢渣-蒙脱土-污泥复合钢渣陶粒,与钢渣-蒙脱土-可溶性淀粉复合陶粒进行对比研究,探讨强化后钢渣陶粒的除磷性能和除磷机理,考察含磷溶液中磷的去除。结果表明,两种钢渣陶粒的最大除磷率均可达到93%,磷酸盐浓度为1 mg/L时,碱浸-污泥强化钢渣陶粒的最佳投加量为0.5 g,显著低于未强化钢渣陶粒(1 g)。扫描电镜显示碱浸-污泥强化钢渣陶粒孔隙更为密集。X射线衍射表明两种陶粒内含多种金属盐,可与水样中磷酸根离子发生反应将其去除。两种陶粒对Langmuir吸附等温方程拟合度最高,碱浸-污泥强化钢渣陶粒吸附饱和时, 最大吸附量(39.18 mg/g)高于未强化钢渣陶粒(19.18 mg/g) 。两种陶粒对准二级动力学模型的拟合度高于准一级动力学模型,表明两种陶粒对磷吸附属于单分子层吸附,以化学吸附为主。Abstract: In order to improve the phosphorus removal effect of steel slag, alkali leaching pretreatment was used and residual sludge was added to strengthen the preparation of alkali leaching slag–montmorillonite–sludge composite steel slag ceramic particles. The phosphorus removal performance and mechanism of enhanced steel slag ceramic particles were discussed, and the removal of phosphorus-containing wastewater was investigated. The results showed that the maximum phosphorus removal rate of the two kinds of steel slag ceramics could reach 93%. When the phosphate concentration was 1 mg/L, the optimal dosage of alkali impregnation–sludge enhanced steel slag ceramics was 0.5 g, which was significantly lower than that of unstrengthened steel slag ceramics (1 g). Scanning electron microscopy (SEM) analysis showed that the pores of alkali impregnation-sludge enhanced steel slag ceramics were denser. X-ray diffraction (XRD) characterization showed that the two ceramics contained a variety of metal salts, which could be removed by reacting with phosphate ions in the water samples. The two kinds of ceramics have the highest fitting degree to Langmuir adsorption isothermal equation, and the maximum adsorption capacity of alkali impregnated sludge strengthened steel slag ceramics is higher than that of unstrengthened steel slag ceramics (Qmax: 39.18 > 19.18 mg). The fit degree of the two models is higher than that of the quasi-first order kinetic model, which indicates that the adsorption of phosphorus by the two kinds of ceramics belongs to monolayer adsorption and is mainly chemisorption.
-
Key words:
- steel slag /
- phosphorus removal /
- alkaline leaching /
- sludge
-
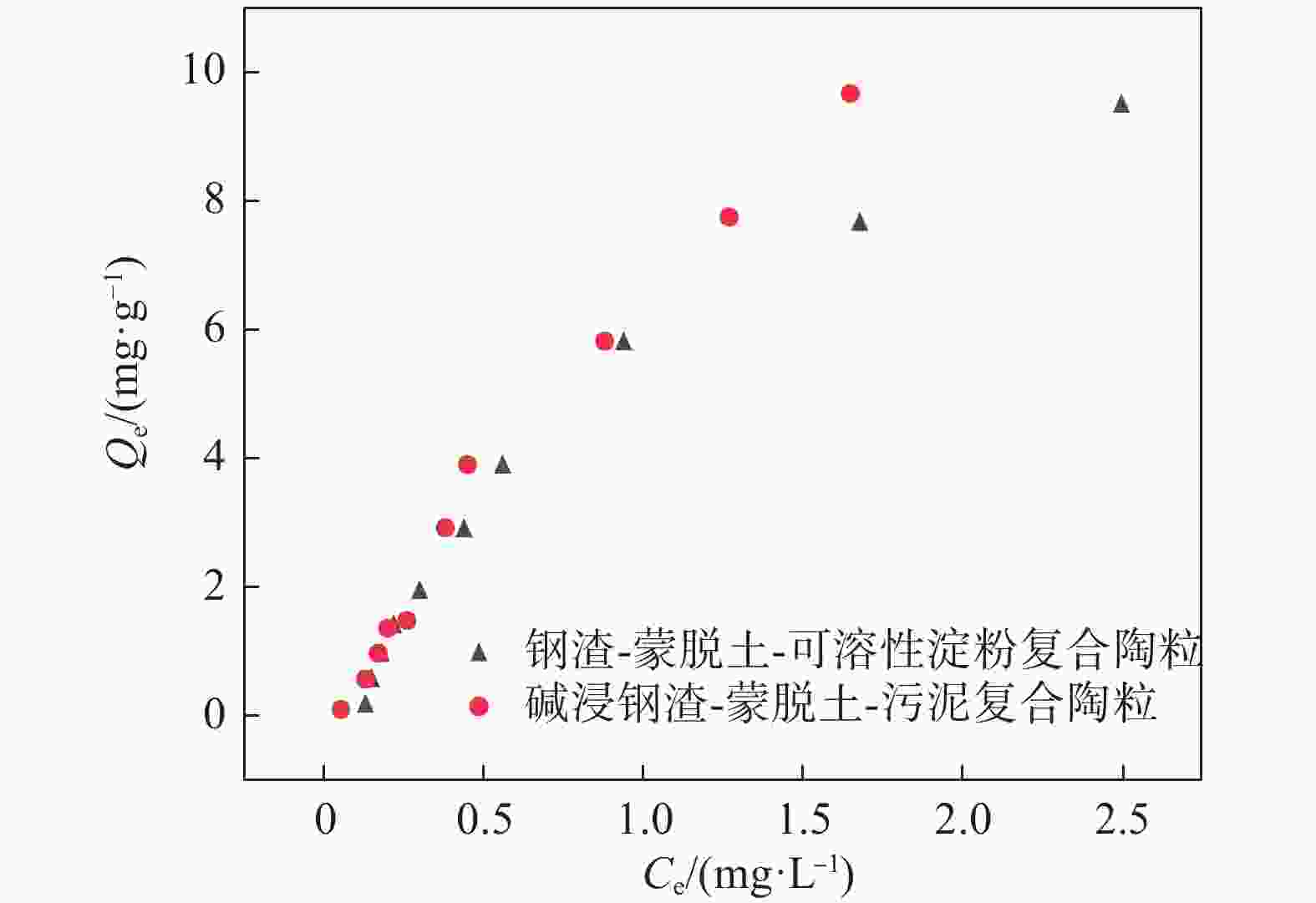
表 1 两种钢渣陶粒的等温吸附方程拟合结果与相关参数
Table 1. Isothermal adsorption equation fitting results and related parameters of two kinds of steel slag ceramides
材料 温度/ ℃ Langmuir模型 Freundlich模型 Qmax/
(L·mg−1)$ {\mathrm{k}}_{\mathrm{L}} $/
(L· mg-1)R2 kF/
(mg1-n·g−1·L-n)$ -\dfrac{1}{\mathrm{n}} $ R2 钢渣-蒙脱土-可溶性淀粉复合陶粒 20 19.18 0.40 0.984 5.07 −0.74 0.961 碱浸钢渣-蒙脱土-污泥复合陶粒 20 39.18 0.20 0.987 6.31 −0.81 0.983 表 2 两种陶粒的动力学吸附方程拟合结果与相关参数
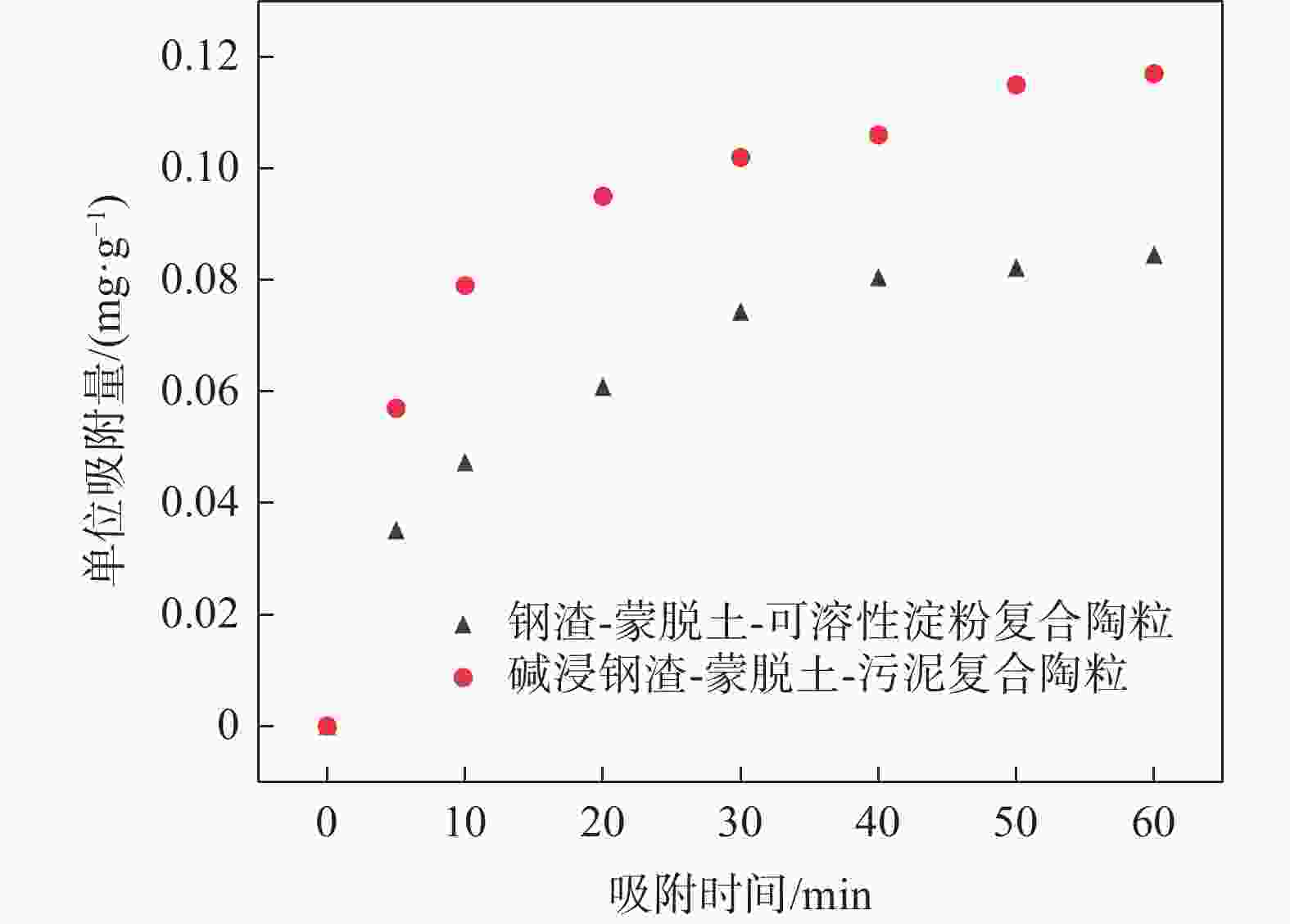
Table 2. Fitting results of kinetic adsorption equations and related parameters of the two ceramides
材料 准一级动力学模型 准二级动力学模型 Qe/
(mg·g−1)k1 /min−1 R2 Qe/
(mg·g−1)k2/[mg·(g·min)−1] R2 钢渣-蒙脱土-可溶性淀粉复合陶粒 0.086 0.065 0.984 0.108 0.615 0.994 碱浸钢渣-蒙脱土-污泥复合陶粒 0.11 0.13 0.979 0.101 1.206 0.994 -
[1] Bai Caiyun, Zhang Chongmiao. Study on preparation of modified steel slag ceramsite and its phosphorus removal performance[J]. Technology of Water Treatment, 2020,46(7):63−66,71. (白彩云, 张崇淼. 改性钢渣陶粒的制备及其除磷性能研究[J]. 水处理技术, 2020,46(7):63−66,71. Bai Caiyun, Zhang Chongmiao . Study on preparation of modified steel slag ceramsite and its phosphorus removal performance[J]. Technology of Water Treatment,2020 ,46 (7 ):63 −66,71 .[2] Akindolie M S, Choi H J. Fe12LaO19 fabricated biochar for removal of phosphorus in water and exploration of its adsorption mechanism[J]. Journal of Environmental Management, 2023,329:117053. doi: 10.1016/j.jenvman.2022.117053 [3] Li J, Huang H, Yu W, et al. A novel lanthanum carbonate for low-level phosphorus removal: Adsorption performance and mechanism[J]. Chemical Engineering Journal, 2023, 473: 145225. [4] Cui Wanying, Ai Hengyu, Zhang Shihao, et al. Research status on application of modified adsorbents in phosphorusremoval from wastewater[J]. Chemical Industry and Engineering Progress, 2020,39(10):17. (崔婉莹, 艾恒雨, 张世豪, 等. 改性吸附剂去除废水中磷的应用研究进展[J]. 化工进展, 2020,39(10):17. Cui Wanying, Ai Hengyu, Zhang Shihao, et al . Research status on application of modified adsorbents in phosphorusremoval from wastewater[J]. Chemical Industry and Engineering Progress,2020 ,39 (10 ):17 .[5] Aigbe U O, Ukhurebor K E, Onyancha R B, et al. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: a review[J]. Journal of Materials Research and Technology, 2021, 14: 2751-2774. [6] Meng Haoyan, Yang Mingfan, Luo Guozhi, et al. Adsorption performance and mechanism of magnetic modified oyster shell powder on phosphorus in water[J]. Chinese Journal of Environmental Engineering, 2021,15(2):446−456. (蒙浩焱, 杨名帆, 罗国芝, 等. 载铁牡蛎壳粉对水中磷的吸附性能及机理[J]. 环境工程学报, 2021,15(2):446−456. Meng Haoyan, Yang Mingfan, Luo Guozhi, et al . Adsorption performance and mechanism of magnetic modified oyster shell powder on phosphorus in water[J]. Chinese Journal of Environmental Engineering,2021 ,15 (2 ):446 −456 .[7] Liu Y, Liu X H, Wang H C, et al. Pyrite coupled with steel slag to enhance simultaneous nitrogen and phosphorus removal in constructed wetlands[J]. Chemical Engineering Journal, 2023, 470: 143944. [8] Lü Shuqing, Tian Shuangchao, Li Hechao, et al. Research status of phosphorus removal by solid waste adsorption[J]. Industrial Water Treatment, 2020,40(5):1−7. (吕淑清, 田双超, 李鹤超, 等. 固体废弃物吸附除磷研究现状[J]. 工业水处理, 2020,40(5):1−7. Lü Shuqing, Tian Shuangchao, Li Hechao, et al . Research status of phosphorus removal by solid waste adsorption[J]. Industrial Water Treatment,2020 ,40 (5 ):1 −7 .[9] Li Zimu, Li Canhua, Zha Yuhong, et al. Study on the adsorption mechanism of Cu2+ by steel slag-manganese slag composite ceramics[J]. Industrial Water Treatment, 2022,42(8):113−119. (李子木, 李灿华, 查雨虹, 等. 钢渣-锰渣复合陶粒对Cu2+的吸附机理研究[J]. 工业水处理, 2022,42(8):113−119. Li Zimu, Li Canhua, Zha Yuhong, et al . Study on the adsorption mechanism of Cu2+ by steel slag-manganese slag composite ceramics[J]. Industrial Water Treatment,2022 ,42 (8 ):113 −119 .[10] Wang Jiabin, Li Xing, Qiu Liping, et al. Preparation of non-sintering composite steel slag filter and its phosphorus adsorption characteristics[J]. China Water & Wastewater, 2019,35(11):86−91. (王嘉斌, 李星, 邱立平, 等. 免烧复合钢渣滤料的制备及其磷吸附特性[J]. 中国给水排水, 2019,35(11):86−91. Wang Jiabin, Li Xing, Qiu Liping, et al . Preparation of non-sintering composite steel slag filter and its phosphorus adsorption characteristics[J]. China Water & Wastewater,2019 ,35 (11 ):86 −91 .[11] Liu Xiao, Li Xuelian, Cao Guoping, et al. Adsorption effect of steel slag haydite on phosphorus in wastewater[J]. Industrial Water Treatment, 2014,34(1):18−21. (刘晓, 李学莲, 曹国凭, 等. 钢渣陶粒对废水中磷的吸附特性[J]. 工业水处理, 2014,34(1):18−21. Liu Xiao, Li Xuelian, Cao Guoping, et al . Adsorption effect of steel slag haydite on phosphorus in wastewater[J]. Industrial Water Treatment,2014 ,34 (1 ):18 −21 .[12] Pei Xuanyuan, Ren Hongyu, Ren Nanqi, et al. Review on the application of sludge derived biochar in the treatment of emerging contaminants in water environment[J]. Water & Wastewater Engineering, 2021,57(S2):545−552. (裴轩瑗, 任宏宇, 任南琪, 等. 污泥生物炭处理水环境新兴污染物研究进展[J]. 给水排水, 2021,57(S2):545−552. Pei Xuanyuan, Ren Hongyu, Ren Nanqi, et al . Review on the application of sludge derived biochar in the treatment of emerging contaminants in water environment[J]. Water & Wastewater Engineering,2021 ,57 (S2 ):545 −552 .[13] Wang Hongbin, Du Yanxia. Progress of application of acid modified fly ash in wastewater treatment[J]. World Nonferrous Metals, 2020(5):204−205. (王宏宾, 杜艳霞. 酸改性粉煤灰在废水处理中的应用研究进展[J]. 世界有色金属, 2020(5):204−205. Wang Hongbin, Du Yanxia . Progress of application of acid modified fly ash in wastewater treatment[J]. World Nonferrous Metals,2020 (5 ):204 −205 .[14] Zhang Jian, Wan Dongjin, Liu Yongde, et al. Alkali treatment of ZSM-5 molecular sieve and its adsorptive performance of Pb2+ from aqueous solution[J]. Journal of Environmental Engineering Technology, 2015(4):277−283. (张健, 万东锦, 刘永德, 等. 碱改性 ZSM-5沸石分子筛吸附去除水种Pb2+的研究[J]. 环境工程技术学报, 2015(4):277−283. Zhang Jian, Wan Dongjin, Liu Yongde, et al . Alkali treatment of ZSM-5 molecular sieve and its adsorptive performance of Pb2+ from aqueous solution[J]. Journal of Environmental Engineering Technology,2015 (4 ):277 −283 .[15] Majid A F A, Dewi R, Shahri N N M, et al. Enhancing adsorption performance of alkali activated kaolinite in the removal of antibiotic rifampicin from aqueous solution[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023: 132209. [16] Cao Jun, Wang Ruochen, Zhu Hualun, et al. Effect of fenton pre-oxidation on the physicochemical properties of sludge-based biochar and its adsorption mechanisms for ammonia nitrogen removal[J]. Journal of Environmental Chemical Engineering, 2023,11(5):110689. doi: 10.1016/j.jece.2023.110689 [17] Xin Wang, Song Yonghui, Zhang Yadi, et al. Research progress of preparation of sewage sludge-basedcarbonaceous adsorbents and their adsorption characteristics[J]. Journal of Environmental Engineering Technology, 2017,7(3):306−317. (辛旺, 宋永会, 张亚迪, 等. 污泥基碳吸附材料的制备及其吸附性能研究进展[J]. 环境工程技术学报, 2017,7(3):306−317. doi: 10.3969/j.issn.1674-991X.2017.03.044 Xin Wang, Song Yonghui, Zhang Yadi, et al . Research progress of preparation of sewage sludge-basedcarbonaceous adsorbents and their adsorption characteristics[J]. Journal of Environmental Engineering Technology,2017 ,7 (3 ):306 −317 . doi: 10.3969/j.issn.1674-991X.2017.03.044[18] Chen Yongliang, Li Huimin, Shi Lei, et al. Adsorption of amoxicillin from water by sludge-rice husk pellet biochar[J]. Technology of Water Treatment, 2023,49(10):64−69. (陈永亮, 李慧敏, 石磊, 等. 污泥-稻壳颗粒生物炭对水中阿莫西林的吸附[J]. 水处理技术, 2023,49(10):64−69. Chen Yongliang, Li Huimin, Shi Lei, et al . Adsorption of amoxicillin from water by sludge-rice husk pellet biochar[J]. Technology of Water Treatment,2023 ,49 (10 ):64 −69 .[19] Xu K, Li L, Huang Z, et al. Efficient adsorption of heavy metals from wastewater on nanocomposite beads prepared by chitosan and paper sludge[J]. Science of the Total Environment, 2022,846:157399. doi: 10.1016/j.scitotenv.2022.157399 [20] Qiao Yong. Study on dephosphorization of converter slag by leaching and removal of phosphorus from leach liquor[D]. Chongqing: Chongqing University, 2017. (谯勇. 转炉钢渣浸出脱磷及含磷浸出液吸附除磷研究[D]. 重庆: 重庆大学, 2017.Qiao Yong. Study on dephosphorization of converter slag by leaching and removal of phosphorus from leach liquor[D]. Chongqing: Chongqing University, 2017. [21] Zhu Dianmei, Shao Bolin, Zhong Keyi, et al. Adsorption performance of lanthanum-modified steel slag towards fluoride ion in water[J]. Chinese Journal of Environmental Engineering, 2023,17(4):1167−1176. (朱殿梅, 邵波霖, 钟可意, 等. 镧改性钢渣对水中氟离子的吸附性能[J]. 环境工程学报, 2023,17(4):1167−1176. Zhu Dianmei, Shao Bolin, Zhong Keyi, et al . Adsorption performance of lanthanum-modified steel slag towards fluoride ion in water[J]. Chinese Journal of Environmental Engineering,2023 ,17 (4 ):1167 −1176 .[22] Ren Z, Liu Y, Yuan L, et al. Optimizing the content of nano-SiO2, nano-TiO2 and nano-CaCO3 in portland cement paste by response surface methodology[J]. Journal of Building Engineering, 2021,35:102073. doi: 10.1016/j.jobe.2020.102073 [23] Wang F P, Liu T J, Cai S, et al. A review of modified steel slag application in catalytic pyrolysis, organic degradation, electrocatalysis, photocatalysis, transesterification and carbon capture and storage[J]. Applied Sciences, 2021,11(10):4539. doi: 10.3390/app11104539 [24] Chen Meiling, Yan Jiabao, Xie Pengkai, et al. Preparation and catalytic performance of steel-making slag and sludgeceramsite catalyst[J]. Journal of Wuhan University of Science and Technology, 2019,42(5):349−353. (陈美玲, 颜家保, 谢鹏凯, 等. 钢渣污泥陶粒催化剂的制备及其催化性能[J]. 武汉科技大学报, 2019,42(5):349−353. Chen Meiling, Yan Jiabao, Xie Pengkai, et al . Preparation and catalytic performance of steel-making slag and sludgeceramsite catalyst[J]. Journal of Wuhan University of Science and Technology,2019 ,42 (5 ):349 −353 .[25] Zhang Yun, Xiao Qing, Xu Shanni, et al. Adsorption studies on phosphate by amino-functionalized nano-size composite materials[J]. Acta Chimica Sinica, 2012,17:1839−1846. (张蕴, 晓青, 许姗妮, 等. 氨基功能化纳米复合材料对磷酸盐的吸附研究[J]. 化学学报, 2012,17:1839−1846. Zhang Yun, Xiao Qing, Xu Shanni, et al . Adsorption studies on phosphate by amino-functionalized nano-size composite materials[J]. Acta Chimica Sinica,2012 ,17 :1839 −1846 .[26] An Q, Miao Y, Zhao B, et al. An alkali modified biochar for enhancing Mn2+ adsorption: Performance and chemical mechanism[J]. Materials Chemistry and Physics, 2020,248:122895. doi: 10.1016/j.matchemphys.2020.122895 [27] Zhang X, Yu J, Jin B, et al. Experimental research on the gaseous PbCl2 adsorption by thermal alkali modified coal fly ash[J]. Journal of Environmental Chemical Engineering, 2022,10(3):107912. doi: 10.1016/j.jece.2022.107912 [28] Ouyang Jia. Preparation and adsorption experiment of phosphate removal ceramsite[D]. Chongqing: Chongqing University, 2017. (欧阳嘉. 新型除磷填料的制备及吸附实验研究[D]. 重庆: 重庆大学, 2017.Ouyang Jia. Preparation and adsorption experiment of phosphate removal ceramsite[D]. Chongqing: Chongqing University, 2017. -




 下载:
下载: