Current situation of resource utilization of waste acid from titanium dioxide production
-
摘要: 钛白废酸中含有可观的资源,废酸的再加工与增值正逐渐成为钛颜料工业的焦点。针对硫酸法钛白副产废酸的回收与利用现状展开综述,主要介绍了废酸在提取金属元素、浸出金属元素、回收硫酸以及工业生产方面的应用,并在此基础上,对今后钛白废酸资源化利用的发展做了相关展望,以期为相关行业的从业者带来废酸利用方面的参考。Abstract: Waste acid after processing titanium dioxide contains valuable resources. The reprocessing and value-added of waste acid is gradually becoming the focus of titanium pigment industry. In this paper, the current situation of recovery and utilization of waste acid from titanium dioxide have been reviewed, mainly focusing on the application of waste acid in extracting metal elements, leaching metal elements, recovering sulfuric acid and industrial production. And then the future development of resource utilization of waste acid from titanium dioxide have been discussed.
-
Key words:
- titanium dioxide /
- sulfuric acid process /
- waste acid /
- resource utilization
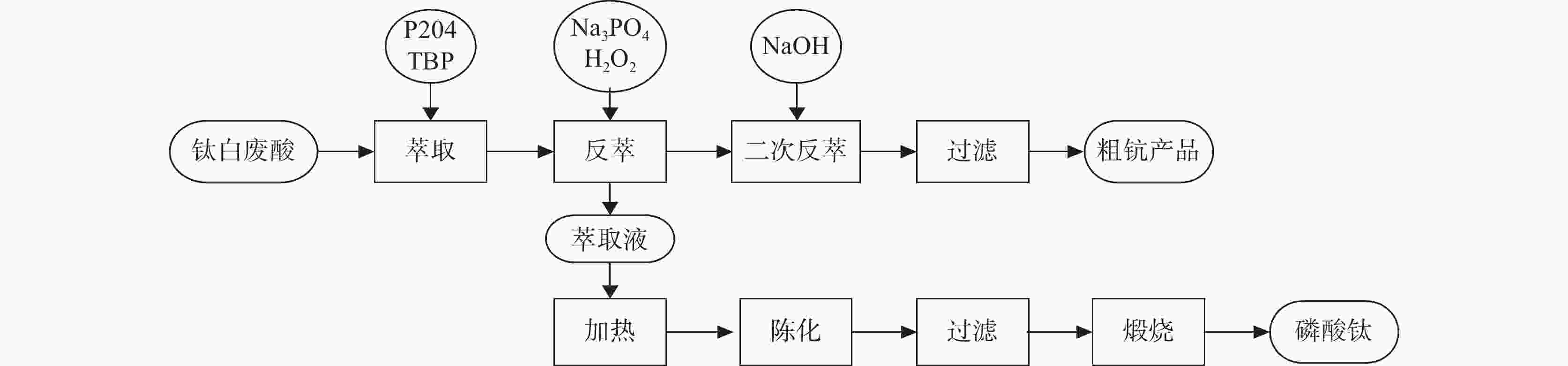
-
表 1 钛白废酸的金属含量
Table 1. Metal contents in waste acid after processing titanium dioxide
mmol/L Fe Ti Mg Al Mn Ca V Sc 87.1 6.6 11.0 7.1 2.8 2.1 0.9 0.03 -
[1] Liang Y, Ding H. Mineral-TiO2 composites: Preparation and application in papermaking, paints and plastics[J]. Journal of Alloys and Compounds, 2020,844:139−156. [2] Feltrin J, De Noni A, Hotza D, et al. Design guidelines for titania-silica-alumina ceramics with tuned anatase to rutile transformation[J]. Ceramics International, 2019,45(5):5179−5188. doi: 10.1016/j.ceramint.2018.12.026 [3] Zheng F Q, Guo Y F, Qiu G Z, et al. A novel process for preparation of titanium dioxide from Ti-bearing electric furnace slag: NH4HF2-HF leaching and hydrolyzing process[J]. Journal of Hazardous Materials, 2018,344:490−498. doi: 10.1016/j.jhazmat.2017.10.042 [4] Luo L P, Wu H Q, Yang J, et al. Effects of microwave pre-treatment on the flotation of ilmenite and titanaugite[J]. Minerals Engineering, 2020,155(15):106452. [5] Sun Zheyu, Xia Yuan, Zhou Lei, et al. Status quo and development of titanium dioxide industry in China[J]. Coating and Protection, 2020,41(7):33−41. (孙哲宇, 夏渊, 周磊, 等. 中国钛白粉行业发展现状分析[J]. 涂层与防护, 2020,41(7):33−41. [6] Hao X L, Lu L, Liang B, et al. Solvent extraction of titanium from the simulated ilmenite sulfuric acid leachate by trialkylphosphine oxide[J]. Hydrometallurgy, 2012,113/114:185−191. doi: 10.1016/j.hydromet.2011.12.023 [7] Fu Yijiang. Performance of China's titanium dioxide industry in 2019 and outlook[J]. China Coatings, 2020,35(5):20−23,41. (付一江. 2019年中国钛白粉行业运行情况及展望[J]. 中国涂料, 2020,35(5):20−23,41. [8] Li Chong, Zhou Jun, Liu Yao, et al. Emission and comprehensive utilization of spent sulfuric acid in China[J]. The Chinese Journal of Process Engineering, 2018,18(S1):24−34. (李崇, 周俊, 刘瑶, 等. 我国废硫酸产生及综合利用现状[J]. 过程工程学报, 2018,18(S1):24−34. doi: 10.12034/j.issn.1009-606X.20180116 [9] Qiu H B, Wang M L, Xie Y M, et al. From trace to pure: Recovery of scandium from the waste acid of titanium pigment production by solvent extraction[J]. Process Safety and Environmental Protection, 2019,121:118−124. doi: 10.1016/j.psep.2018.10.027 [10] Li Y H, Li Q G, Zhang G Q, et al. Separation and recovery of scandium and titanium from spent sulfuric acid solution from the titanium dioxide production process[J]. Hydrometallurgy, 2018,178:1−6. doi: 10.1016/j.hydromet.2018.01.019 [11] Li Yuhua, Li Qinggang, Zhang Guiqing, et al. Recycling utilization of scandium from hydrolyzed sulfuric acid of titanium dioxide[J]. CIESC Journa, 2017,68(7):2818−2825. (李玉华, 李青刚, 张贵清, 等. 钛白水解废酸中钪的回收[J]. 化工学报, 2017,68(7):2818−2825. [12] Zou D, Li H L, Chen J, et al. Recovery of scandium from spent sulfuric acid solution in titanium dioxide production using synergistic solvent extraction with D2EHPA and primary amine N1923[J]. Hydrometallurgy, 2020,197:105463. doi: 10.1016/j.hydromet.2020.105463 [13] Zhu Xiaobo, Niu Zepeng, Li Wang, et al. Experimental study on recovery of vanadium from titanium dioxide wastewater by solvent[J]. Rare Metals and Cemented Carbides, 2020,48(1):9−12. (朱晓波, 牛泽鹏, 李望, 等. 溶剂萃取法回收钛白废液中钒的实验研究[J]. 稀有金属与硬质合金, 2020,48(1):9−12. [14] Ma Xueyang, Liang Bing, Lv Li, et al. Recovery of titanium by solvent extraction from waste sulfuric acid discharged in titanium dioxide production[J]. Iron Steel Vanadium Titanium, 2016,37(4):62−68. (马雪阳, 梁斌, 吕莉, 等. 从钛白废酸中萃取回收钛[J]. 钢铁钒钛, 2016,37(4):62−68. [15] Zhang W G, Zhang T G, Li T G, et al. Basic research on the leaching behavior of vanadium-bearing steel slag with titanium white waste acid[J]. Journal of Environmental Chemical Engineering, 2021,9(1):104897. doi: 10.1016/j.jece.2020.104897 [16] Zhang G Q, Zhang T A, Lu G Z, et al. Extraction of vanadium from LD converter slag by pressure leaching process with titanium white waste acid[J]. Rare Metal Materials and Engineering, 2015,44(8):1894−1898. doi: 10.1016/S1875-5372(15)30120-X [17] Xiong Y, Wu B, Zhu J W, et al. Preparation of magnesium hydroxide from leachate of dolomitic phosphate ore with dilute waste acid from titanium dioxide production[J]. Hydrometallurgy, 2014,142:137−144. doi: 10.1016/j.hydromet.2013.11.013 [18] Ma Guangqiang, Zou Min, Xia Dong. Experimental study on titanium concentrate leaching to prepare Ti-rich material with titanium[J]. Inorganic Chemicals Industry, 2016,48(8):67−69. (马光强, 邹敏, 夏冬. 钛白废酸加压浸出钛精矿制备富钛料实验研究[J]. 无机盐工业, 2016,48(8):67−69. [19] Wang Jihua, Gao Jianming. Leaching of copper fom pyrite cinder using titanium white waste acid[J]. Hydrometallurgy of China, 2020,39(2):100−103. (王吉华, 高建明. 用钛白废酸从硫酸渣中浸出铜[J]. 湿法冶金, 2020,39(2):100−103. [20] Fan Yanjin, He Hangjun, Zhang Jianfei, et al. Study on technology of scandium oxide extraction from red mud and titanium white waste acid[J]. Nonferrous Metals(Extractive Metallurgy), 2015,(5):55−57. (樊艳金, 何航军, 张建飞, 等. 钛白废酸与赤泥联合提取氧化钪的工艺研究[J]. 有色金属(冶炼部分), 2015,(5):55−57. [21] Feng X, Jiang L Y, Song Y. Titanium white sulfuric acid concentration by direct contact membrane distillation[J]. Chemical Engineering Journal, 2016,285:101−111. doi: 10.1016/j.cej.2015.09.064 [22] Si Z T, Han D, Gu J, et al. Exergy analysis of a vacuum membrane distillation system integrated with mechanical vapor recompression for sulfuric acid waste treatment[J]. Applied Thermal Engineering, 2020,178:115516. doi: 10.1016/j.applthermaleng.2020.115516 [23] Ashoor B B, Mansour S, Giwa A, et al. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review[J]. Desalination, 2016,398:222−246. doi: 10.1016/j.desal.2016.07.043 [24] Feng X, Wu P, Jiang L Y. Titanium white waste acid concentration by DCMD: Wetting, crystallization, and fouling[J]. Desalination, 2018,440:161−174. doi: 10.1016/j.desal.2018.01.009 [25] Hu B, Ouyang J T, Jiang L Y. Influence of flocculant polyacrylamide on concentration of titanium white waste acid by direct contact membrane distillation[J]. Chinese Journal of Chemical Engineering, 2020,28(9):2483−2496. doi: 10.1016/j.cjche.2020.03.022 [26] Pang H Y, Lu R F, Zhang T, et al. Chemical dehydration coupling multi-effect evaporation to treat waste sulfuric acid in titanium dioxide production process[J]. Chinese Journal of Chemical Engineering, 2020,28(4):1162−1170. doi: 10.1016/j.cjche.2020.02.009 [27] Yadav V, Raj S K, Rathod N H, et al. Polysulfone/graphene quantum dots composite anion exchange membrane for acid recovery by diffusion dialysis[J]. Journal of Membrane Science, 2020,611:118331. doi: 10.1016/j.memsci.2020.118331 [28] Zhang X J, Zhang F, Liu M H, et al. Quaternized poly(2, 6-dimethyl-1, 4-phenylene oxide)s with zwitterion groups as diffusion dialysis membranes for acid recovery[J]. Separation and Purification Technology, 2020,250:117267. doi: 10.1016/j.seppur.2020.117267 [29] Liu Shuli, Zheng Yajie, Zhang Shouchun. Recovery of sulfuric acid from titanium white waste acid by diffusion dialysis[J]. Technology of Water Treatment, 2017,43(2):67−70,84. (刘淑莉, 郑雅杰, 张寿春. 扩散渗析法回收钛白废酸中的硫酸[J]. 水处理技术, 2017,43(2):67−70,84. [30] Xu T W, Yang W H. Sulfuric acid recovery from titanium white (pigment) waste liquor using diffusion dialysis with a new series of anion exchange membranes — static runs[J]. Journal of Membrane Science, 2001,183(2):193−200. doi: 10.1016/S0376-7388(00)00590-1 [31] Agrawal A, Sahu K K. An overview of the recovery of acid from spent acidic solutions from steel and electroplating industries[J]. Journal of Hazardous Materials, 2009,171(1/3):61−75. [32] Chen F, Wang X M, Liu W Z, et al. Selective extraction of nitric and acetic acids from etching waste acid using N235 and MIBK mixtures[J]. Separation and Purification Technology, 2016,169:50−58. doi: 10.1016/j.seppur.2016.06.008 [33] Shin C H, Kim J Y, Kim J Y, et al. A solvent extraction approach to recover acetic acid from mixed waste acids produced during semiconductor wafer process[J]. Journal of Hazardous Materials, 2009,162(2/3):1278−1284. [34] Ren X L, Wei Q F, Chen Y X, et al. Utilization of the dilute acidic sulfate effluent as resources by coupling solvent extraction–oxidation–hydrolysis[J]. Journal of Hazardous Materials, 2015,299:702−710. doi: 10.1016/j.jhazmat.2015.08.003 [35] Wei Q F, Ren X L, Guo J J, et al. Recovery and separation of sulfuric acid and iron from dilute acidic sulfate effluent and waste sulfuric acid by solvent extraction and stripping[J]. Journal of Hazardous Materials, 2016,304:1−9. doi: 10.1016/j.jhazmat.2015.10.049 [36] Hao Rongqing, Yan Yongqing. Review of circular economy pattern for S−P−Ti industry chain[J]. Sulphur Phosphorus & Bulk Materials Handling Related Engineering, 2011,(4):39−46,52. (郝荣清, 严永清. 硫−磷−钛产业链循环经济模式的评述[J]. 硫磷设计与粉体工程, 2011,(4):39−46,52. doi: 10.3969/j.issn.1009-1904.2011.04.011 [37] Bao Shutao. Industrial practice of efficient and high-value utilization of spent acid generated during titanium dioxide production[J]. Sulphuric Acid Industry, 2014,(6):20−24. (鲍树涛. 钛白废酸高效高值利用工业实践[J]. 硫酸工业, 2014,(6):20−24. doi: 10.3969/j.issn.1002-1507.2014.06.007 [38] 陈玲. 钛白粉生产硫酸废液中铁的脱除过程研究[D]. 上海: 华东理工大学, 2015.Chen Ling. Study on removal of iron from the waste sulfuric acid generated during the production of TiO2 with sulfuric acid [D]. Shanghai: East China University of Science and Technology, 2015. [39] Ji Luojun. Review and prospect of thiophosate industry of China in ten years[J]. Sulphuric Acid Industry, 2017,(8):4−17. (纪罗军. 我国硫磷钛工业十年回顾及展望[J]. 硫酸工业, 2017,(8):4−17. doi: 10.3969/j.issn.1002-1507.2017.08.002 [40] Gavalas S, Gagaoudakis E, Katerinopoulou D, et al. Vanadium oxide nanostructured thin films prepared by aerosol spray pyrolysis for gas sensing and thermochromic applications[J]. Materials Science in Semiconductor Processing, 2019,89:116−120. doi: 10.1016/j.mssp.2018.09.008 [41] Wang Xin. Comprehensive utilization of waste acid from by-product of titanium dioxide production by sulfuric acid process[J]. Henan Science and Technology, 2017,(15):136−137. (王新. 硫酸法钛白粉生产副产品废酸的综合利用[J]. 河南科技, 2017,(15):136−137. doi: 10.3969/j.issn.1003-5168.2017.15.062 [42] Yu Wang, Zheng Yajie. Effect of recrystallization on formation of α-Fe2O3 particles prepared from ferrous sulphate by hydrothermal process[J]. Journal of Central South University(Science and Technology), 2016,47(9):2951−2957. (余旺, 郑雅杰. 硫酸亚铁的重结晶对其水热法制备α-Fe2O3粒子的影响[J]. 中南大学学报(自然科学版), 2016,47(9):2951−2957. doi: 10.11817/j.issn.1672-7207.2016.09.007 [43] 周娟. 七水合硫酸亚铁在H2SO4-HCl-H2O体系中的溶解度测定及相变研究[D]. 北京: 北京化工大学, 2018.Zhou Juan. Phase transition of FeSO4·7H2O to FeSO4·H2O in the H2SO4-HCl-H2O system by modeling solubility [D]. Beijing: Beijing University of Chemical Technology, 2018. [44] Wu Jianchun, Lu Ruifang. Study on preparation of FeSO4·H2O from titanium white waste acid and FeSO4·7H2O[J]. Inorganic Chemicals Industry, 2018,50(6):75−77. (吴健春, 路瑞芳. 钛白副产废酸和七水硫酸亚铁制备一水硫酸亚铁的研究[J]. 无机盐工业, 2018,50(6):75−77. [45] Zhen Mingkai, Yang Weixue. Study on producing yellow ferric oxide with by-product copperas of titanium white and waste acid[J]. China Resources Comprehensive Utilization, 2011,29(8):22−25. (郑明凯, 杨为学. 利用钛白副产绿矾和废酸制备氧化铁黄的研究[J]. 中国资源综合利用, 2011,29(8):22−25. doi: 10.3969/j.issn.1008-9500.2011.08.003 [46] Ke P C, Song K Z, Ghahreman A, et al. Improvement of scorodite stability by addition of crystalline polyferric sulfate[J]. Hydrometallurgy, 2019,185:162−172. doi: 10.1016/j.hydromet.2019.02.012 [47] Gong Jiazhu, Wu Ninglan, Lu Xiangfang, et al. Research progress of recovery and utilization technology of waste sulphuric acid from titanium dioxide[J]. Sulphuric Acid Industry, 2019,(12):6−9. (龚家竹, 吴宁兰, 陆祥芳, 等. 钛白粉废硫酸回收利用技术研究进展[J]. 硫酸工业, 2019,(12):6−9. doi: 10.3969/j.issn.1002-1507.2019.12.002 [48] Tao Houdong, Ma Zhengcheng, Qi Fei. Industrial practice of high efficiency and high value utilization of titanium white waste acid[J]. China Resources Comprehensive Utilization, 2019,37(5):79−81. (陶厚东, 马征程, 齐飞. 钛白废酸高效高值利用工业实践[J]. 中国资源综合利用, 2019,37(5):79−81. doi: 10.3969/j.issn.1008-9500.2019.05.025 -




 下载:
下载: