Recent progress on V2O5 nanowire nonwovens preparation and application in advanced electrochemical energy storage devices
-
摘要: 五氧化二钒纳米纤维作为重要的钒基功能材料,在电化学储能领域有着重要应用。通过分析一维五氧化二钒纳米纤维及其聚集态材料在先进电化学储能器件应用领域的研究进展,结合近期研究前沿比较了不同五氧化二钒纳米纤维布合成方法的优缺点,认为采用降低尺寸和增加比表面积的策略将使其在电化学储能方面有更好的性能。同时,展望了V2O5纳米纤维布在未来先进电化学储能领域的发展前景,提出了其主要的发展和研究方向。Abstract: As an important V-based functional material, vanadium pentoxide (V2O5) nanofiber has a significant application in the field of electrochemical energy storage. We herein gave an overview of the one-dimensional (1D) V2O5-based materials for the application of advanced electrochemical energy storage and conversion, especially regarding the advantages and disadvantages of different synthetic methods of V2O5 nanowire nonwovens combined with recent research frontiers. It was considered that reducing the size and increasing the specific surface area will endow V2O5 with better performance in this field. Meanwhile, the development prospect of V2O5 nanofiber cloth in the field of advanced electrochemical energy storage in the future as well as the main development and research direction was provided.
-
0. 引言
我国特别是攀西地区钒资源极其丰富,是钒资源储量和钒产品大国[1],发展高附加值钒产品不仅有利于产业结构调整升级,也有利于发展国家经济、增强国力。五氧化二钒(V2O5)是最重要的钒功能材料和中间体,在特种钢、玻璃陶瓷工业着色剂、硫酸石化工业催化剂以及电化学储能材料等领域都有重要应用价值[2-4]。五氧化二钒纳米材料的结构有零维、一维、二维、三维。与二维相比,一维五氧化二钒纳米线及其聚集体由于比表面积大、活性点位多以及良好的机械柔韧性,对柔性智能器件特别是先进电化学储能器件的开发有独特的优势[5]。目前合成一维五氧化二钒纳米材料的方法主要有水热法[6-9]、模板法[10-12]、溶胶凝胶法[13-14]、溶解/重结晶[15-16]以及其他一些化学、物理方法等[5]。五氧化二钒纳米线编织无纺布由于其特殊宏观形貌和微观结构有更广泛而特殊的应用。文献报道可以将制备得到的V2O5纳米线经过二次抽滤或蒸发方法得到纳米纤维布[6-7,12,17-18],或者通过静电纺丝钒类前驱体复合物并经煅烧得到较大面积的V2O5纤维无纺布[5,19]。但其低成本大规模制备困难限制了其进一步应用。开发低成本、性能优良的五氧化二钒纳米纤维布制备技术对先进电化学储能器件的广泛应用并进一步拓展应用领域有重要价值。
1. 制备方法、结构与性能
目前制备V2O5纳米纤维及其无纺布(或称为纤维膜或陶瓷膜)的方法普遍成本高、制备周期长或者工艺复杂。绝大多数方法采用纯度较高的偏钒酸铵(AMV)或V2O5为原料制备氧化钒纳米纤维,这些原料成本较高。常规的制备方法中,水热法需要高温高压(一般180 ℃以上)、反应时间长(一般十几小时以上),难以大规模生产。溶胶凝胶法一般需要采用阳离子交换树脂,成本高、工艺复杂或制备周期长(一般几天以上)。沉淀法需要额外加入矿化剂、反应周期长(一般几天时间)。模板法和静电纺丝法存在工艺复杂、成本高并且辅助剂的加入导致纤维强度低等问题,同时所得纤维结构受模板或造孔剂的影响。总体上,V2O5纳米纤维单根结构基本类似,长径比有所不同。此外,V2O5纳米纤维可组装成多种聚集态,例如纤维布、多孔泡沫等。
文献[2-5]系统介绍了低维钒基氧化物纳米材料的合成及其组成结构,其在能源存储与转化用自支撑电极及固态电池方面展现出独特的优势。例如,Zhang等[6]以V2O5粉末为原料在双氧水体系中230 ℃水热反应12 h,经去离子水/乙醇洗涤、真空干燥和空气退火(400 ℃,3 h)得到V2O5超长纳米纤维。延长反应时间也可得到厘米级超长纳米纤维[9];但如果反应温度不够高,所得产品会含有一定量结合水,形成水合五氧化二钒[7-8]。Wang等[8]采用硝酸体系水热反应(180 ℃,24 h)并经过热处理(400 ℃,200 min)制备得到V2O5纤维布。
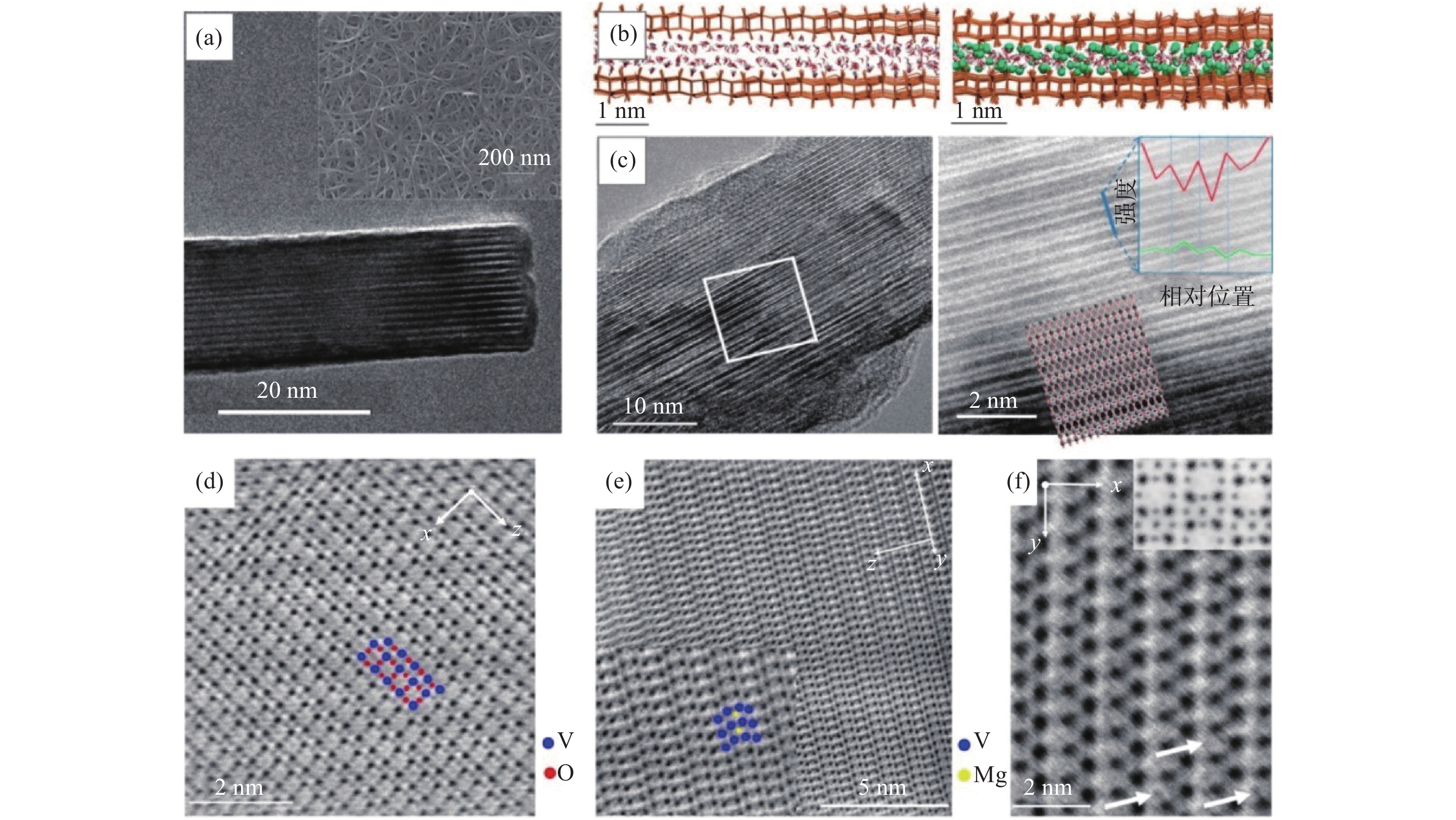
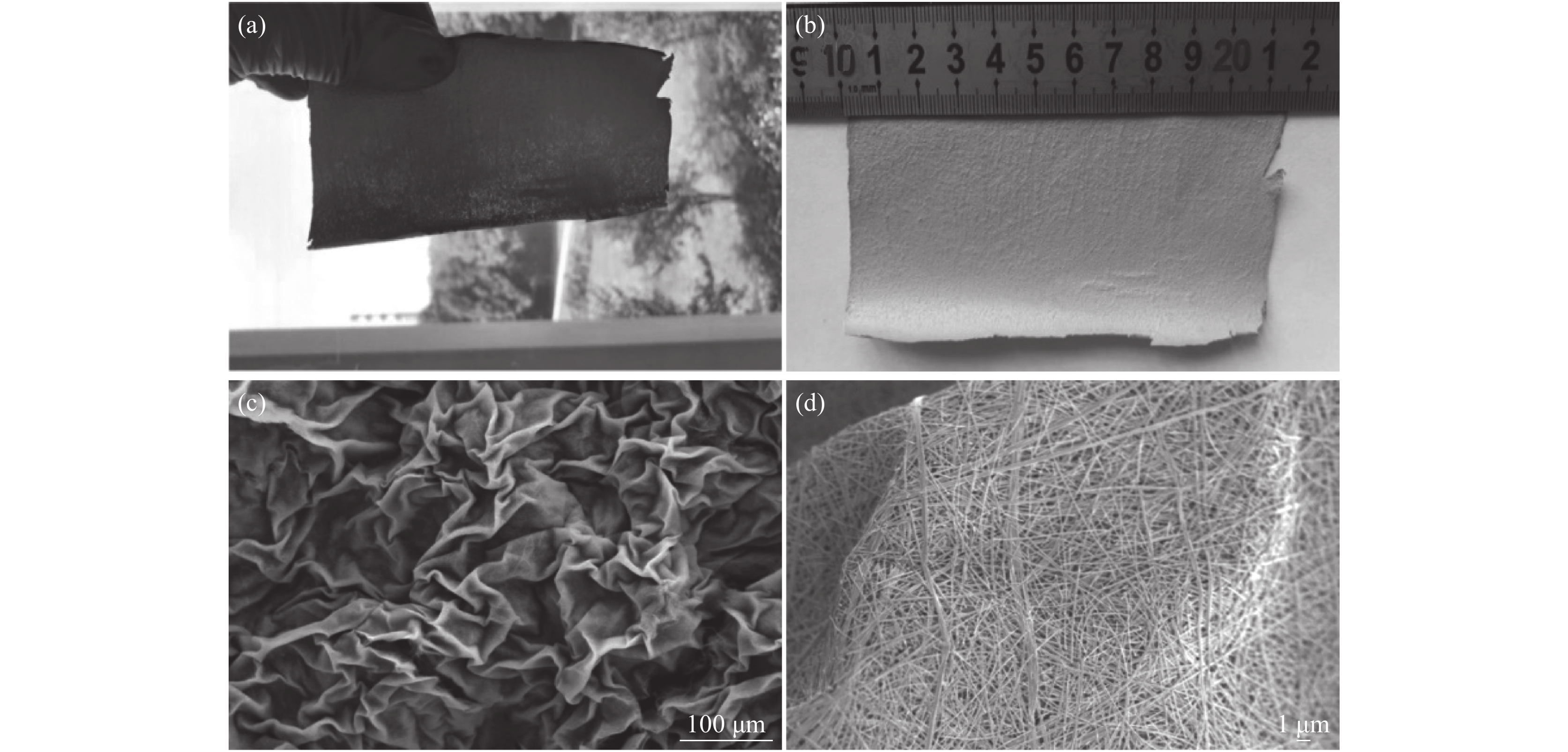
笔者等[20]提出一种制备纳米纤维多褶皱无纺布的方法,以廉价工业级AMV和盐酸一步法制备大尺寸高纯五氧化二钒纳米纤维多褶皱无纺布。该工艺采用的技术方案机理和调控关键主要在于控制五氧化二钒纳米纤维在反应釜内壁表面的成核、稳定生长以及纤维的交错排布过程。所得产品宏观和微观形貌如图1所示。
经过技术革新,可以以温和的条件超快速大规模低成本制备得到氧化钒纳米纤维,以及多种异形纤维、海绵钒氧化物及其潜在衍生物聚集体(如由异形纳米纤维组成的轻质通孔粉体、膜、块体)[21]。该项技术采用更初级、价格低廉的工业级钒前驱体多钒酸胺(APV)、简单工艺(分散、搅拌、过滤)、超短周期(几分钟)、室温常压(最低能耗)制备氧化钒纳米纤维无纺布及其聚集体,有重要的技术价值和市场前景,为后续低成本、高附加值产品研发提供关键技术支撑。
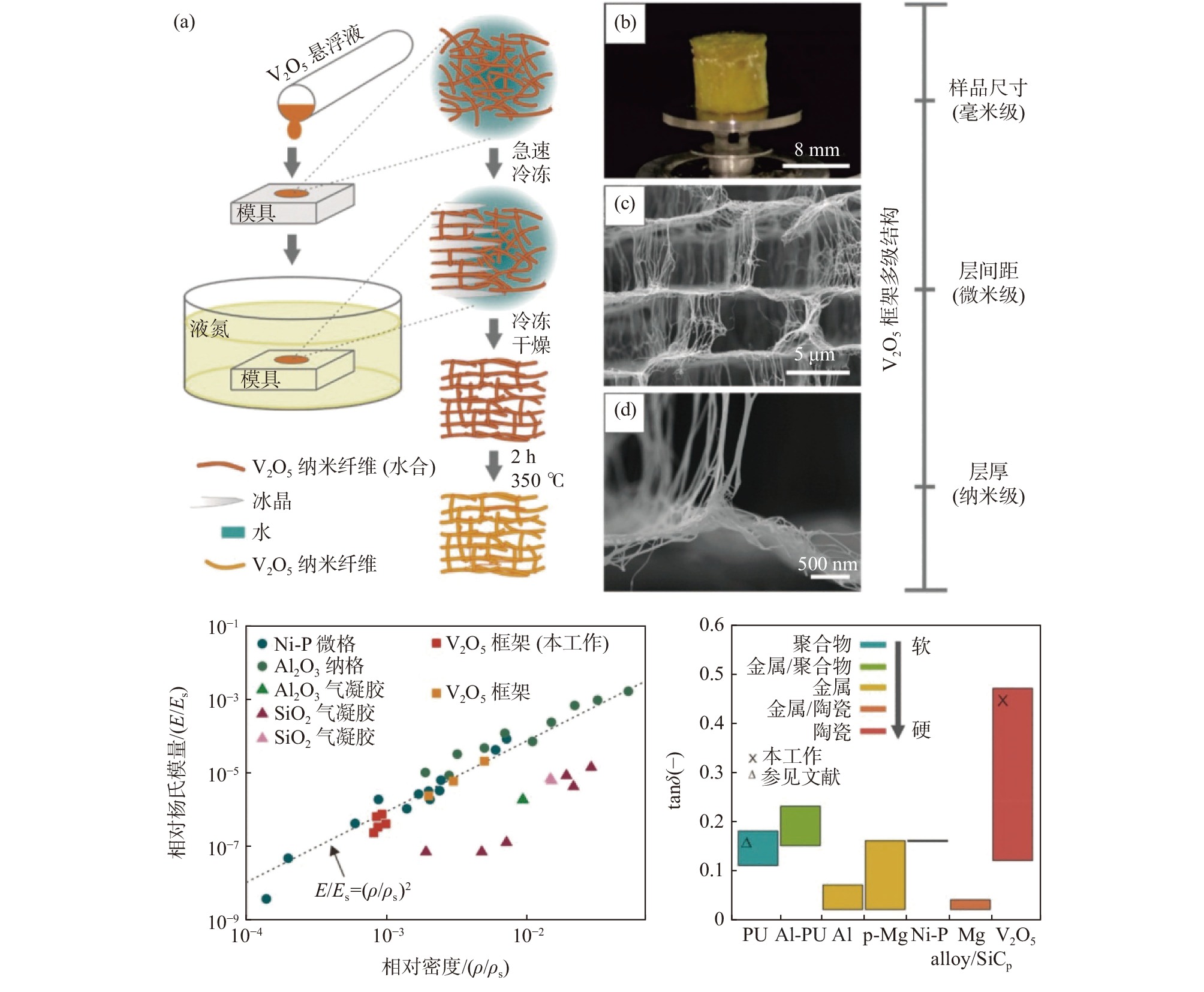
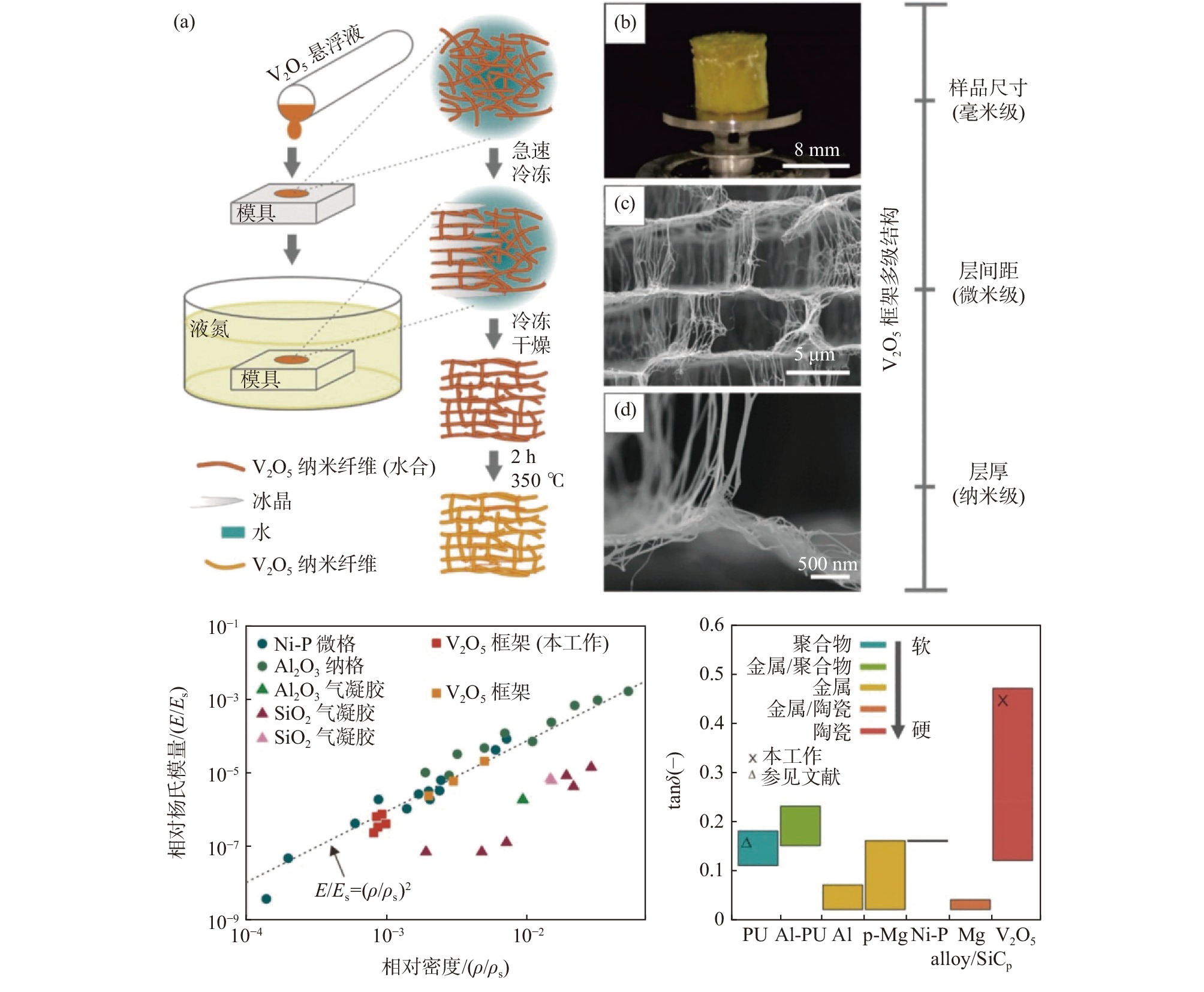
与二维纤维布[7,18-19,22-23]不同的是,一维纳米纤维也可以通过自组装形成强韧的三维海绵结构。例如通过急速冷却和冷冻干燥可得到超轻五氧化二钒纳米纤维多孔泡沫材料(大孔柔性层柱结构,类墨鱼骨),通过外部模具和内部形成的冰晶模板可方便调控宏观和微观形貌[24-26]。该材料孔隙率高达99.9%,密度仅3 mg/cm3(类似气凝胶),与传统陶瓷材料不同的是,其在低压缩段具有超高阻尼性能(或减震性能,类似聚氨酯泡沫),见图2。有催化、传感器、储能等多方面潜在应用。
 图 2 高有序V2O5纳米纤维泡沫制备、结构及其优异阻尼性能Figure 2. Formation, structure, and performances (relative Young’s modulus and damping capacities) of single V2O5 nanofibers based highly ordered all-ceramic scaffolds[24]
图 2 高有序V2O5纳米纤维泡沫制备、结构及其优异阻尼性能Figure 2. Formation, structure, and performances (relative Young’s modulus and damping capacities) of single V2O5 nanofibers based highly ordered all-ceramic scaffolds[24]2. 电化学储能应用
由于一维五氧化二钒纤维在光学、电子和电化学等方面的特殊性质,其有很多独特的应用,如电致变色[10,27]、电化学储能[5](锂离子电池[6,28-29]、铝离子电池、锌离子电池[30]、锂硫电池[31-32]、超级电容器[33-34])、传感器[14,35-36]、催化剂、电磁屏蔽、场发射器件、光电器件以及其他纳米器件(制动器[37])等[9,38]。后面就电化学储能做重点概述。
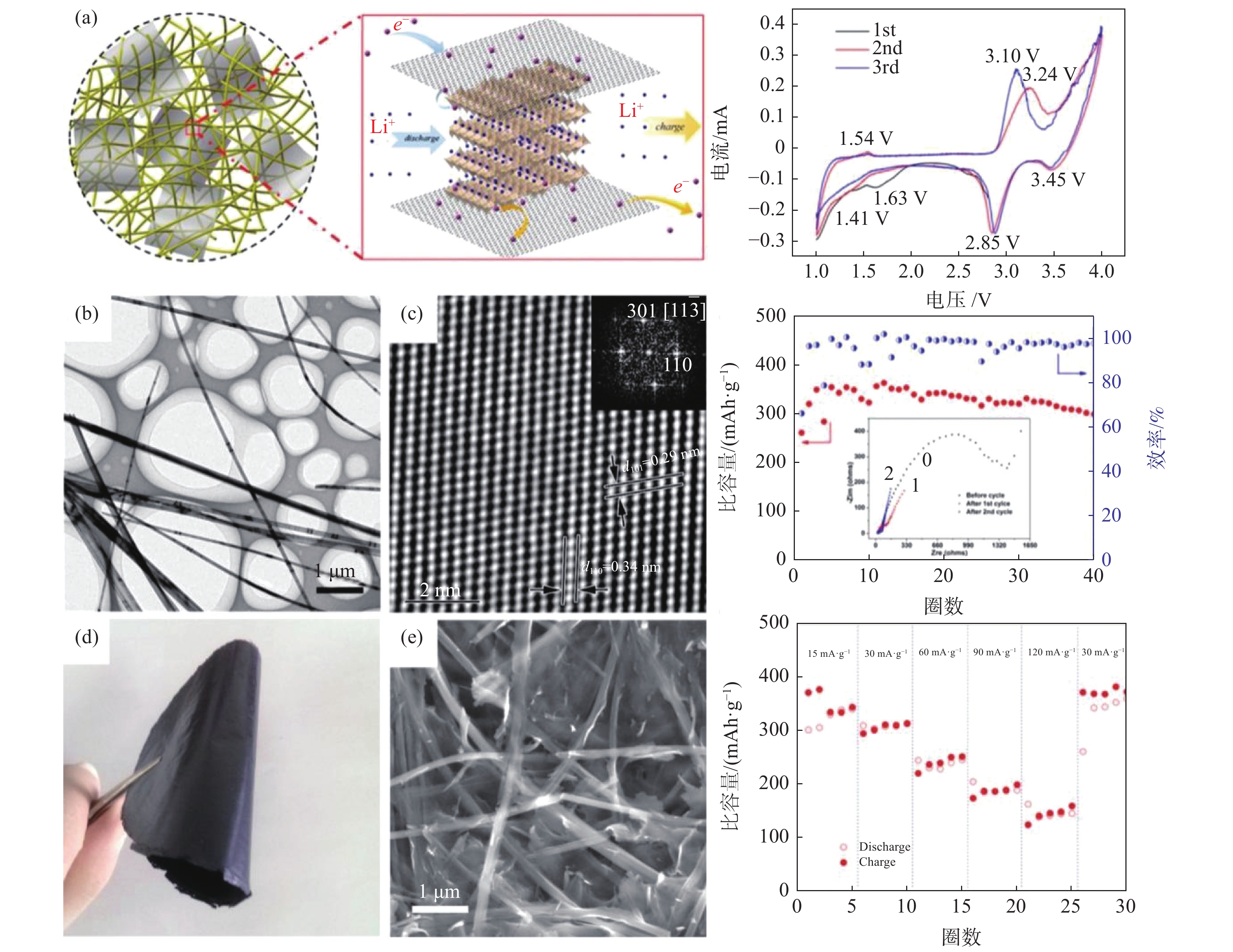
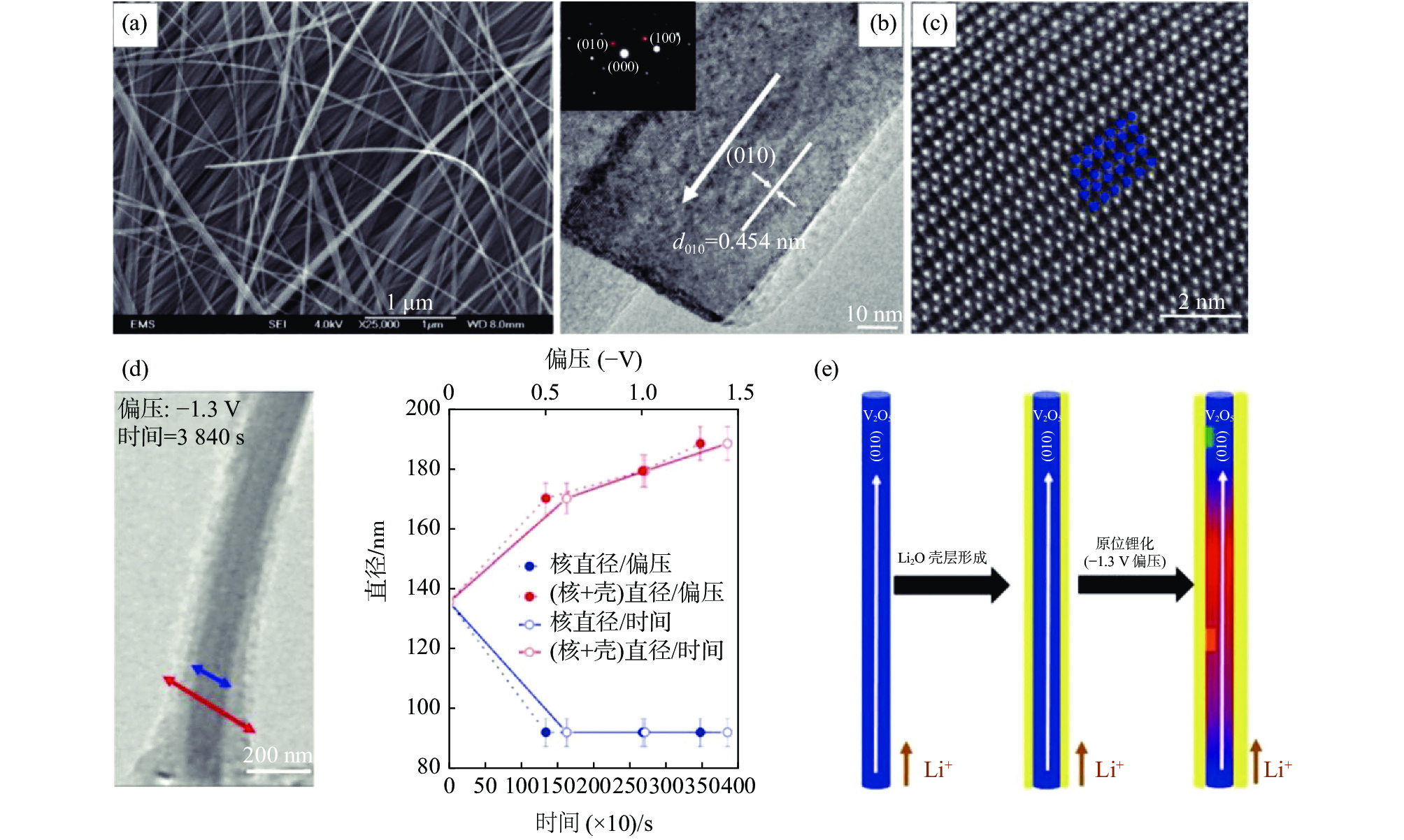
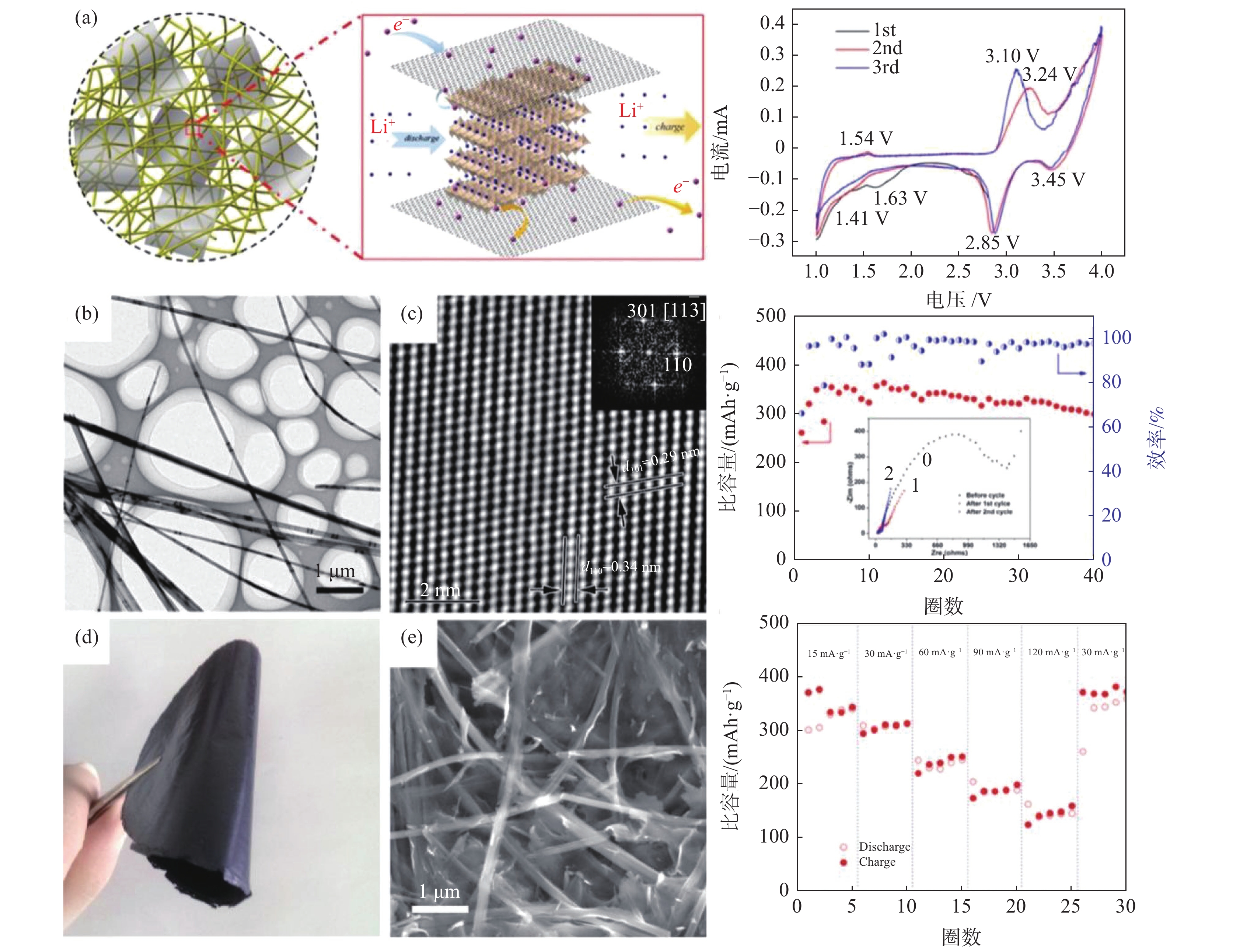
V2O5为层状结构[5-6,28],理论容量高达443 mAh/g,比能量可达1550 Wh/kg,远高于目前商业化传统锂离子电池(LIBs)正极材料(原位表征可通过透射电镜(TEM)[39]或扫描透射X射线显微镜(STXM)[40]实现,其中原位透射电镜如图3 [13,41]所示)。一维材料有独特的载流子传输和柔性编织优势[5]。导电组分或骨架的复合/杂化或元素掺杂可以提升其电导率、离子扩散系数以及提升结构稳定性,从而实现高倍率比容量和高比能量并增强循环稳定性[5,19,42],例如石墨烯骨架(2%质量比即可实现V2O5纳米带/石墨烯干凝胶杂化正极材料的近理论容量、长稳定循环和高倍率)[13],为先进电池研发提供重要借鉴。通过电极和电解液优化,如采用石墨烯、碳纳米管、导电聚合物等轻质柔性集流体/集成电极和优选固态电解质,可制备柔性[7,15,41,43]甚至全固态储能器件(图4)[5-6,44-45],为高安全性可穿戴设备保驾护航。通过特定的层层自组装方法,可以实现透明储能器件的制备[46]。此外,过渡金属化合物作为锂硫电池夹层(interlayer)对提升其循环稳定性和使用寿命有重要作用,杂化夹层的引入可以显著增强多硫化物物理化学吸附能力,促进硫化物的催化转化,提升电子和离子传输扩散。V2O5纳米纤维膜对锂硫电池中的多硫化物有很好的吸附、催化转化和离子电导率增强作用,Guo等采用V2O5纳米纤维/石墨烯夹层使锂硫电池单圈容量衰减率降低至0.041%(1000圈循环),且可实现高硫负载量(5.5 mg/cm2)和高面积容量(>4 mAh/cm2)[32]。Chen等通过在商业多孔隔膜上负载V2O5纳米纤维复合层(聚苯胺同轴外壳),锂硫电池的倍率性能和循环性能得到显著提升,1 C比容量达到942 mAh/g,5 C比容量可达670 mAh/g,1000圈的容量衰减率仅为每圈0.037%[31]。
 图 3 V2O5纳米纤维层状晶格结构、原位锂化表征及储锂机理Figure 3. Structural and lithiation progression characterizations of the pristine V2O5 nanowire as well as the proposed lithiation mechanism[39]
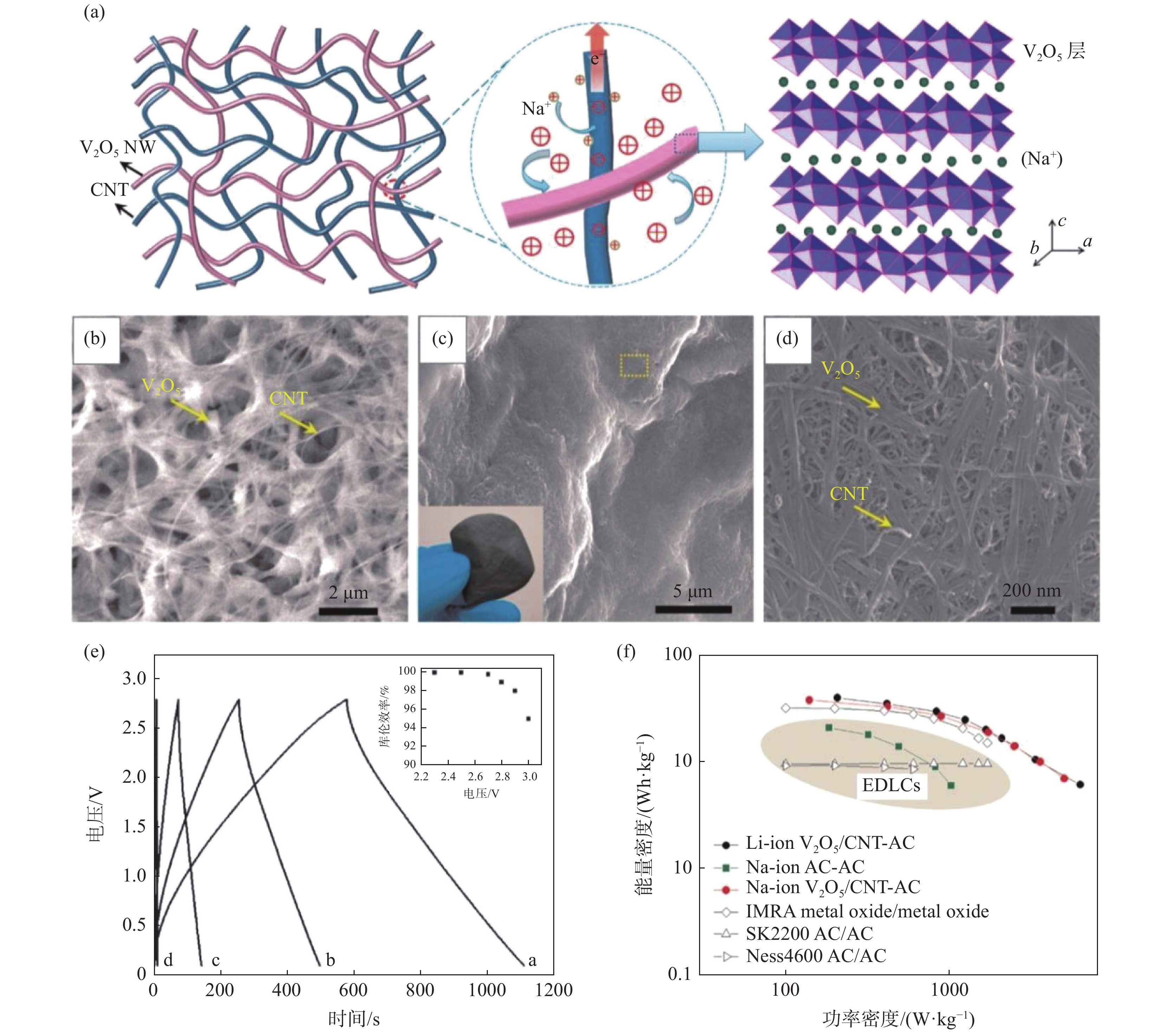
图 3 V2O5纳米纤维层状晶格结构、原位锂化表征及储锂机理Figure 3. Structural and lithiation progression characterizations of the pristine V2O5 nanowire as well as the proposed lithiation mechanism[39]由于锂离子电池所用的锂资源储量有限,后锂离子电池时代已逐渐拉开帷幕。包括钠离子、钾离子、镁离子、铝离子、钙离子、锌离子电池等基于富金属储量的电池的研究逐渐火热[5,19,47-49]。V2O5及其衍生物的可调层间距为较大离子半径或多价金属离子提供了插嵌/脱嵌平台,例如镁离子电池(MIBs)中镁负极体积容量可达3833 mAh/cm3(高于锂金属负极的2 046 mAh/cm3),镁离子在层状V2O5中的存在状态如图5所示[48]。而铝离子电池(AIBs)由于其多电子传输特质和高理论比容量/功率密度(约442 mAh/g,3000 W/kg)优势得到广泛研究。而V2O5具有层状晶体结构,为Al3+离子提供了良好的可逆插嵌和脱除基质,透射电镜研究发现其存在二相转变反应过程(即V5+离子还原形成无定型层及形成新相),这与Li+或Na+单纯的插嵌/脱除反应不同[47]。并且研究发现分级纤维结构V2O5基电极电化学性能明显高于其他形态V2O5或碳基正极材料(图6)[26,50]。材料尺寸、层间结构及电导性(即增强离子和电子传输动力学)是主要的优化方向。但新型可充电电池多存在电压平台较低或循环稳定性较差等问题,随着这些关键参数的优化,将来可与锂离子电池媲美,并在成本和安全性上展现出更多优势。
 图 4 V2O5纳米纤维及其自支撑柔性石墨烯复合膜结构及其全固态锂离子电池正极材料性能(80℃)Figure 4. Structure of V2O5-nanowire, freestanding flexible rGO/V2O5 composite paper and the performance of the as-prepared all-solid-state lithium–vanadium (rGO/V2O5/PEO-MIL-53(Al)-LiTFSI/Li) battery[6]
图 4 V2O5纳米纤维及其自支撑柔性石墨烯复合膜结构及其全固态锂离子电池正极材料性能(80℃)Figure 4. Structure of V2O5-nanowire, freestanding flexible rGO/V2O5 composite paper and the performance of the as-prepared all-solid-state lithium–vanadium (rGO/V2O5/PEO-MIL-53(Al)-LiTFSI/Li) battery[6] 图 6 高有序V2O5纳米纤维泡沫衍生膜及其铝离子电池性能Figure 6. Structures of the creased highly porous scaffold comprised of V2O5 nanowires and its aluminum-ion batteries (AIBs) performances[26]
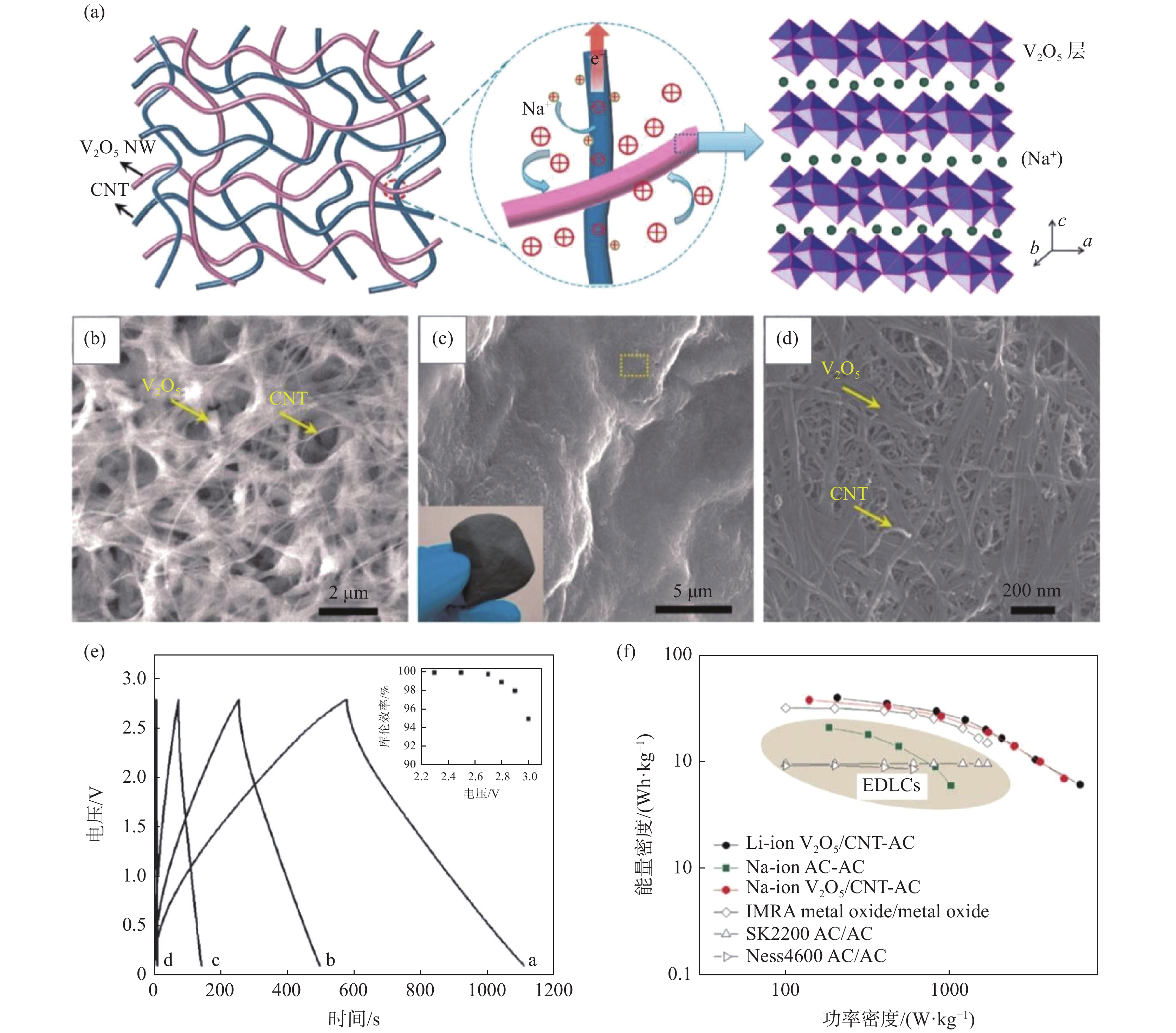
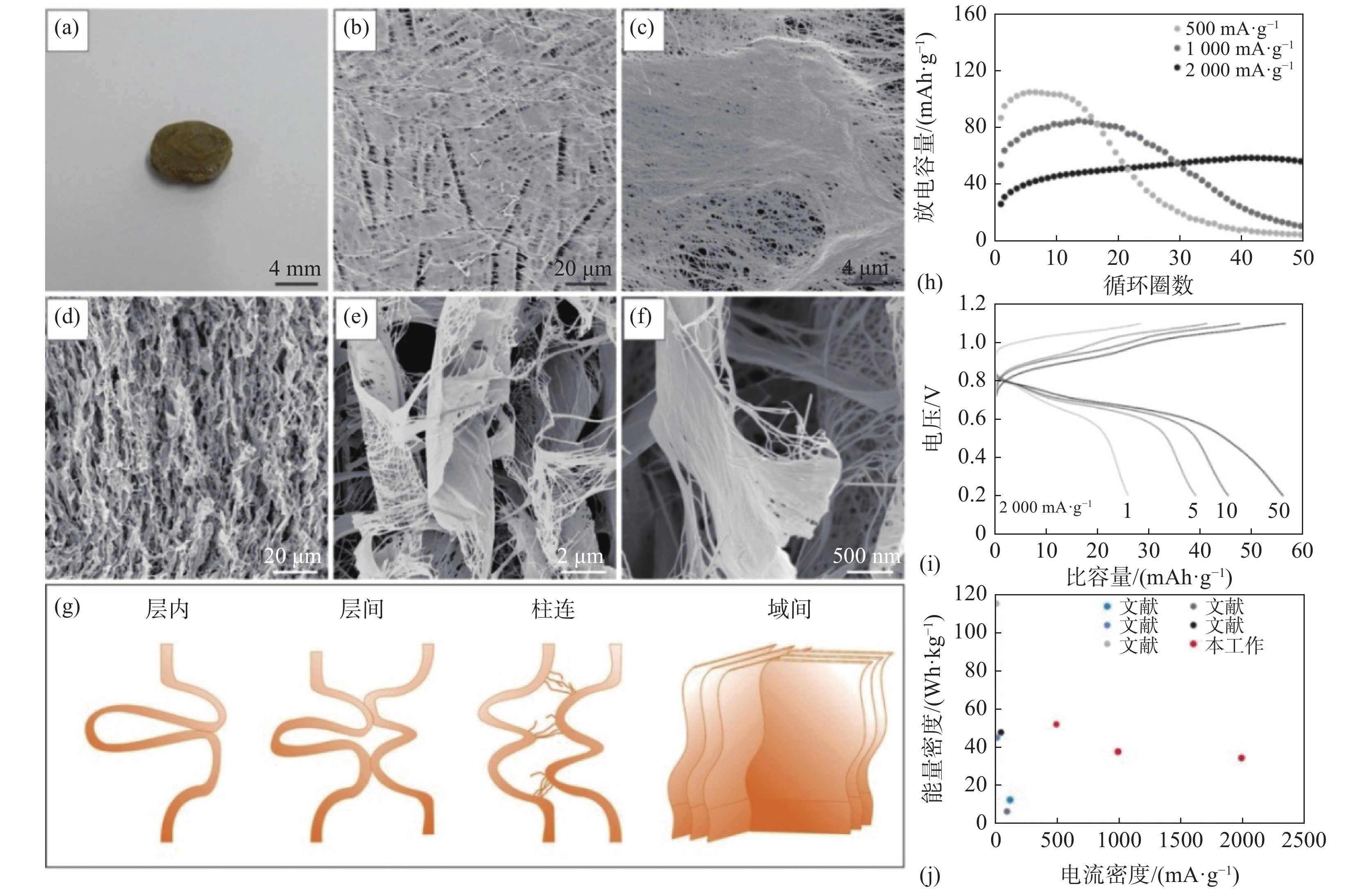
图 6 高有序V2O5纳米纤维泡沫衍生膜及其铝离子电池性能Figure 6. Structures of the creased highly porous scaffold comprised of V2O5 nanowires and its aluminum-ion batteries (AIBs) performances[26]电容器相比电池具有更大的功率密度和更优异的循环性能。特别是赝电容型(或称杂化型或非对称型)超级电容器与电池同属电化学储能器件,具有电池和电容器的双重优势,这得益于电极材料的巨大比表面积和快速表面电化学反应动力学。例如V2O5可与碳纳米管共湿纺成杂化纤维,可用作柔性超级电容器电极材料。设计的全固态柔性非对称超级电容器具有优异的能量密度和柔韧性,为可穿戴储能器件提供重要借鉴[33]。相比水基电容器,有机电解液的采用能大幅提升超级电容器的电压及能量密度(E=1/2 CV2,E为能量密度,C为电容,V为电压,即电压提升为2倍,能量密度提升为4倍),例如锂离子[51]或钠离子[52]非对称有机电容器,可以同时实现高功率密度和高能量密度以及优异的循环稳定性(锂离子非对称有机电容器1万圈电容保持率约80%)[51]。V2O5纳米纤维/碳纳米管复合膜及其钠离子非对称超级电容器性能见图7。
 图 7 V2O5纳米纤维/碳纳米管复合膜及其钠离子非对称超级电容器性能Figure 7. Structures of the layered-V2O5-nanowires/CNTs nanocomposite film and the performance of the as-prepared Na-ion asymmetric supercapacitor[52]
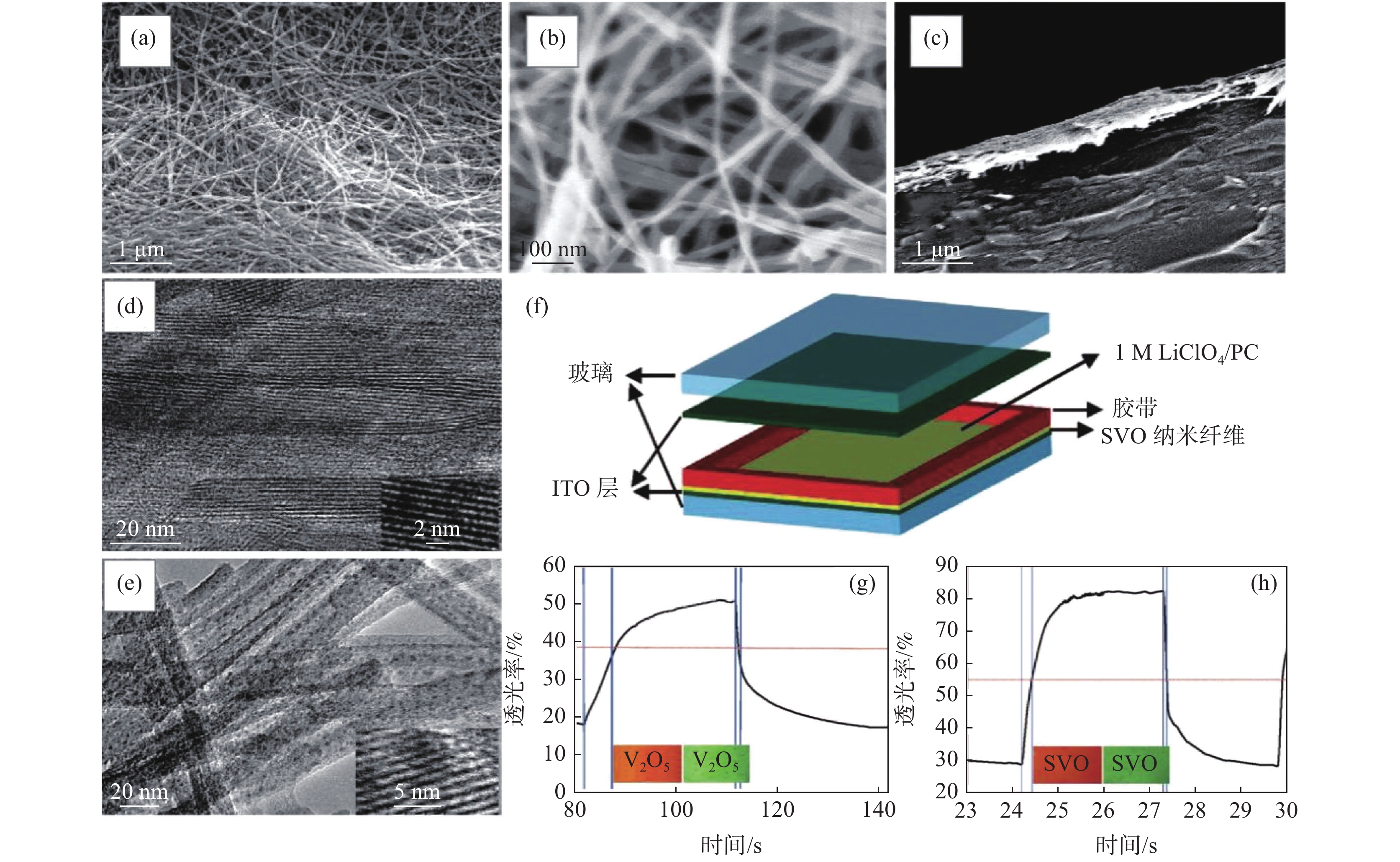
图 7 V2O5纳米纤维/碳纳米管复合膜及其钠离子非对称超级电容器性能Figure 7. Structures of the layered-V2O5-nanowires/CNTs nanocomposite film and the performance of the as-prepared Na-ion asymmetric supercapacitor[52]此外,V2O5纳米纤维膜也可用于其他领域,比如电致变色,该原理类似于电化学储能/转化。例如V2O5纳米纤维自支撑膜电导率达到0.08 S/cm,经过掺杂(如银掺杂,SVO)可获得更好的电导率和离子扩散效率,电致变色更灵敏(仅需0.2 s),透光率达到60%(图8)[10]。通过进一步同轴涂层修饰,V2O5纳米纤维的电致变色循环稳定性和电容可进一步提升。例如MnO2包覆的V2O5纳米纤维薄膜1000圈的电容保有量可以提升约20%,达到93.6%[27]。
 图 8 V2O5纳米纤维膜及其银掺杂衍生物(SVO)的结构和电致变色性能Figure 8. Structures of the V2O5 nanowire thin film and its Ag-doped derivative (SVO) as well as their electrochromic performance[10]
图 8 V2O5纳米纤维膜及其银掺杂衍生物(SVO)的结构和电致变色性能Figure 8. Structures of the V2O5 nanowire thin film and its Ag-doped derivative (SVO) as well as their electrochromic performance[10]3. 展望
五氧化二钒由于其特殊的层状结构和电子特性,在能量转化和电子器件领域有重要应用。虽然五氧化二钒纳米纤维无纺布结构设计和制备业已取得很多进展和突破,但一些实际应用中的关键问题仍待解决。比如水热法对控制结构形貌相对更有效,但高温高压限制了其大规模制备。因此一步法制备技术和室温常压合成技术将是很有前景的研发方向。此外,其更多特性和应用领域有待进一步发掘。
未来五氧化二钒纳米纤维无纺布在电化学储能领域主要的研究或发展方向包括:
1)开发更简易、可靠、低成本和环境友好的适合大批量制备的技术和工艺。
2)更多地研究材料微观结构,包括层间特性、结构缺陷和晶面堆叠等。
3)进一步理论计算/模拟和原位/实时观测电化学储能及其集成应用的机制,构建纳米结构-性能关系,为材料设计和器件优化服务。
4)柔性、可穿戴电极及储能和电致变色器件的开发。
-
图 2 高有序V2O5纳米纤维泡沫制备、结构及其优异阻尼性能
Figure 2. Formation, structure, and performances (relative Young’s modulus and damping capacities) of single V2O5 nanofibers based highly ordered all-ceramic scaffolds[24]
图 3 V2O5纳米纤维层状晶格结构、原位锂化表征及储锂机理
Figure 3. Structural and lithiation progression characterizations of the pristine V2O5 nanowire as well as the proposed lithiation mechanism[39]
图 4 V2O5纳米纤维及其自支撑柔性石墨烯复合膜结构及其全固态锂离子电池正极材料性能(80℃)
Figure 4. Structure of V2O5-nanowire, freestanding flexible rGO/V2O5 composite paper and the performance of the as-prepared all-solid-state lithium–vanadium (rGO/V2O5/PEO-MIL-53(Al)-LiTFSI/Li) battery[6]
图 6 高有序V2O5纳米纤维泡沫衍生膜及其铝离子电池性能
Figure 6. Structures of the creased highly porous scaffold comprised of V2O5 nanowires and its aluminum-ion batteries (AIBs) performances[26]
图 7 V2O5纳米纤维/碳纳米管复合膜及其钠离子非对称超级电容器性能
Figure 7. Structures of the layered-V2O5-nanowires/CNTs nanocomposite film and the performance of the as-prepared Na-ion asymmetric supercapacitor[52]
图 8 V2O5纳米纤维膜及其银掺杂衍生物(SVO)的结构和电致变色性能
Figure 8. Structures of the V2O5 nanowire thin film and its Ag-doped derivative (SVO) as well as their electrochromic performance[10]
-
[1] Gao Yongzhang. Vanadium resources and its supply and demand situation in China[J]. China Min Mag, 2019,28(S2):5−10. (高永璋. 中国钒矿资源及供需形势分析[J]. 中国矿业, 2019,28(S2):5−10. doi: 10.12075/j.issn.1004-4051.2019.S2.040 [2] Liu P, Zhu K, Gao Y, et al. Recent progress in the applications of vanadium-based oxides on energy storage: from low-dimensional nanomaterials synthesis to 3D micro/nano-structures and free-standing electrodes fabrication[J]. Adv Energy Mater, 2017,7:1700547. doi: 10.1002/aenm.201700547 [3] Liu M, Su B, Tang Y, et al. Recent advances in nanostructured vanadium oxides and composites for energy conversion[J]. Adv Energy Mater, 2017,7:1700885. doi: 10.1002/aenm.201700885 [4] Yue Y, Liang H. Micro- and nano-structured vanadium pentoxide (V2O5) for electrodes of lithium-ion batteries[J]. Adv Energy Mater, 2017,7:1602545. doi: 10.1002/aenm.201602545 [5] Yao J H, Li Y W, Masse R C, et al. Revitalized interest in vanadium pentoxide as cathode material for lithium-ion batteries and beyond[J]. Energy Storage Mater, 2018,11:205−259. doi: 10.1016/j.ensm.2017.10.014 [6] Zhang Y, Lai J, Gong Y, et al. A safe high-performance all-solid-state lithium–vanadium battery with a freestanding V2O5 nanowire composite paper cathode[J]. ACS Appl Mater Interfaces, 2016,8:34309−34316. doi: 10.1021/acsami.6b10358 [7] Rui X, Zhu J, Liu W, et al. Facile preparation of hydrated vanadium pentoxide nanobelts based bulky paper as flexible binder-free cathodes for high-performance lithium ion batteries[J]. RSC Adv, 2011,1:117−122. doi: 10.1039/c1ra00281c [8] Wang Y, Zhang H J, Siah K W, et al. One pot synthesis of self-assembled V2O5 nanobelt membrane via capsule-like hydrated precursor as improved cathode for Li-ion battery[J]. J Mater Chem, 2011,21:10336−10341. doi: 10.1039/c1jm10783f [9] Zhai T, Liu H, Li H, et al. Centimeter-long V2O5 nanowires: from synthesis to field-emission, electrochemical, electrical transport, and photoconductive properties[J]. Adv Mater, 2010,22:2547−2552. doi: 10.1002/adma.200903586 [10] Xiong C R, Aliev A E, Gnade B, et al. Fabrication of silver vanadium oxide and V2O5 nanowires for electrochromics[J]. ACS Nano, 2008,2:293−301. doi: 10.1021/nn700261c [11] Chou S L, Wang J Z, Sun J Z, et al. High capacity, safety, and enhanced cyclability of lithium metal battery using a V2O5 nanomaterial cathode and room temperature ionic liquid electrolyte[J]. Chem Mater, 2008,20:7044−7051. doi: 10.1021/cm801468q [12] Ding N, Liu S, Feng X, et al. Hydrothermal growth and characterization of nanostructured vanadium-based oxides[J]. Cryst Growth Des, 2009,9:1723−1728. doi: 10.1021/cg800645c [13] Liu Q, Li Z F, Liu Y, et al. Graphene-modified nanostructured vanadium pentoxide hybrids with extraordinary electrochemical performance for Li-ion batteries[J]. Nat Commun, 2015,6:6127. doi: 10.1038/ncomms7127 [14] Biette L, Carn F, Maugey M, et al. Macroscopic fibers of oriented vanadium oxide ribbons and their application as highly sensitive alcohol microsensors[J]. Adv Mater, 2005,17:2970−2974. doi: 10.1002/adma.200501368 [15] Rui X, Tang Y, Malyi O I, et al. Ambient dissolution–recrystallization towards large-scale preparation of V2O5 nanobelts for high-energy battery applications[J]. Nano Energy, 2016,22:583−593. doi: 10.1016/j.nanoen.2016.03.001 [16] Lausser C, Cölfen H, Antonietti M. Mesocrystals of vanadium pentoxide: a comparative evaluation of three different pathways of mesocrystal synthesis from tactosol precursors[J]. ACS Nano, 2011,5:107−114. doi: 10.1021/nn1017186 [17] Wang P P, Yao Y X, Xu C Y, et al. Self-standing flexible cathode of V2O5 nanobelts with high cycling stability for lithium-ion batteries[J]. Ceram Int, 2016,42:14595−14600. doi: 10.1016/j.ceramint.2016.06.075 [18] Burghard Z, Leineweber A, van Aken P A, et al. Hydrogen-bond reinforced vanadia nanofiber paper of high stiffness[J]. Adv Mater, 2013,25:2468−2473. doi: 10.1002/adma.201300135 [19] Armer C F, Yeoh J S, Li X, et al. Electrospun vanadium-based oxides as electrode materials[J]. J Power Sources, 2018,395:414−429. doi: 10.1016/j.jpowsour.2018.05.076 [20] 倪伟. 高纯五氧化二钒纳米纤维无纺布的制备方法: 中国专利, CN113481656A[P].2021-10-08.Ni Wei. Preparation method of high-purity vanadium pentoxide nanowire non-woven fabrics: China Patent, CN113481656A[P].2021-10-08 . [21] 倪伟. 一种异形氧化钒纳米纤维及其聚集体的低成本室温快速批量制备方法、设备: 中国专利, CN114293321A[P]. 2022-04-08.Ni Wei. Low-cost, room-temperature, rapid and large-scale preparation method and equipment for special-shaped vanadium oxide nanowires and their assemblages: China Patent, CN114293321A[P]. 2022-04-08. [22] Knöller A, Lampa C P, Cube Fv, et al. Strengthening of ceramic-based artificial nacre via synergistic interactions of 1D vanadium pentoxide and 2D graphene oxide building blocks[J]. Sci Rep, 2017,7:40999. doi: 10.1038/srep40999 [23] Wicklein B, Diem A M, Knöller A, et al. Dual-fiber approach toward flexible multifunctional hybrid materials[J]. Adv Funct Mater, 2018,28:1704274. doi: 10.1002/adfm.201704274 [24] Knöller A, Kilper S, Diem A M, et al. Ultrahigh damping capacities in lightweight structural materials[J]. Nano Lett, 2018,18:2519−2524. doi: 10.1021/acs.nanolett.8b00194 [25] Knöller A, Runčevski T, Dinnebier R E, et al. Cuttlebone-like V2O5 nanofibre scaffolds – advances in structuring cellular solids[J]. Sci Rep, 2017,7:42951. doi: 10.1038/srep42951 [26] Diem A M, Bill J, Burghard Z. Creasing highly porous V2O5 scaffolds for high energy density aluminum-ion batteries[J]. ACS Appl Energy Mater, 2020,3:4033−4042. doi: 10.1021/acsaem.0c00455 [27] Sajitha S, Aparna U, Deb B. Ultra-thin manganese dioxide-encrusted vanadium pentoxide nanowire mats for electrochromic energy storage applications[J]. Adv Mater Interfaces, 2019,6:1901038. doi: 10.1002/admi.201901038 [28] Mai L, Xu X, Xu L, et al. Vanadium oxide nanowires for Li-ion batteries[J]. J Mater Res, 2011,26:2175−2185. doi: 10.1557/jmr.2011.171 [29] Zhou Y, Pan Q, Zhang J, et al. Insights into synergistic effect of acid on morphological control of vanadium oxide: toward high lithium storage[J]. Adv Sci, 2021,8:2002579. doi: 10.1002/advs.202002579 [30] Qin X, Wang X, Sun J, et al. Polypyrrole wrapped V2O5 nanowires composite for advanced aqueous zinc-ion batteries[J]. Front Energy Res, 2020,8:199. doi: 10.3389/fenrg.2020.00199 [31] Chen K, Zhang G, Xiao L, et al. Polyaniline encapsulated amorphous V2O5 nanowire-modified multi-functional separators for lithium–sulfur batteries[J]. Small Methods, 2021,5:2001056. doi: 10.1002/smtd.202001056 [32] Guo Y, Zhang Y, Zhang Y, et al. Interwoven V2O5 nanowire/graphene nanoscroll hybrid assembled as efficient polysulfide-trapping-conversion interlayer for long-life lithium–sulfur batteries[J]. J Mater Chem A, 2018,6:19358−19370. doi: 10.1039/C8TA06610H [33] Li H, He J, Cao X, et al. All solid-state V2O5-based flexible hybrid fiber supercapacitors[J]. J Power Sources, 2017,371:18−25. doi: 10.1016/j.jpowsour.2017.10.031 [34] Dong J, Jiang Y, Wei Q, et al. Strongly coupled pyridine-V2O5·nH2O nanowires with intercalation pseudocapacitance and stabilized layer for high energy sodium ion capacitors[J]. Small, 2019,15:1900379. doi: 10.1002/smll.201900379 [35] Leroy C M, Achard M F, Babot O, et al. Designing nanotextured vanadium oxide-based macroscopic fibers: application as alcoholic sensors[J]. Chem Mater, 2007,19:3988−3999. doi: 10.1021/cm0711966 [36] Qi X, Lu Z, You E M, et al. Nanocombing effect leads to nanowire-based, in-plane, uniaxial thin films[J]. ACS Nano, 2018,12:12701−12712. doi: 10.1021/acsnano.8b07671 [37] Gu G, Schmid M, Chiu P W, et al. V2O5 nanofibre sheet actuators[J]. Nat Mater, 2003,2:316−319. doi: 10.1038/nmat880 [38] Myung S, Lee M, Kim G T, et al. Large-scale “surface-programmed assembly” of pristine vanadium oxide nanowire-based devices[J]. Adv Mater, 2005,17:2361−2364. doi: 10.1002/adma.200500682 [39] Mukherjee A, Ardakani H A, Yi T, et al. Direct characterization of the Li intercalation mechanism into α-V2O5 nanowires using in-situ transmission electron microscopy[J]. Appl Phys Lett, 2017,110:213903. doi: 10.1063/1.4984111 [40] De Jesus L R, Horrocks G A, Liang Y, et al. Mapping polaronic states and lithiation gradients in individual V2O5 nanowires[J]. Nat Commun, 2016,7:12022. doi: 10.1038/ncomms12022 [41] Aliahmad N, Liu Y, Xie J, et al. V2O5/graphene hybrid supported on paper current collectors for flexible ultrahigh-capacity electrodes for lithium-ion batteries[J]. ACS Appl Mater Interfaces, 2018,10:16490−16499. doi: 10.1021/acsami.8b02721 [42] Huang X, Rui X, Hng H H, et al. Vanadium pentoxide-based cathode materials for lithium-ion batteries: morphology control, carbon hybridization, and cation doping[J]. Part Part Syst Charact, 2015,32:276−294. doi: 10.1002/ppsc.201400125 [43] Zhang Y, Wang Y, Xiong Z, et al. V2O5 nanowire composite paper as a high-performance lithium-ion battery cathode[J]. ACS Omega, 2017,2:793−799. doi: 10.1021/acsomega.7b00037 [44] Seng K H, Liu J, Guo Z P, et al. Free-standing V2O5 electrode for flexible lithium ion batteries[J]. Electrochem Commun, 2011,13:383−386. doi: 10.1016/j.elecom.2010.12.002 [45] Wang L, Shu T, Guo S, et al. Fabricating strongly coupled V2O5@PEDOT nanobelts/graphene hybrid films with high areal capacitance and facile transferability for transparent solid-state supercapacitors[J]. Energy Storage Mater, 2020,27:150−158. doi: 10.1016/j.ensm.2020.01.026 [46] Gittleson F S, Hwang D, Ryu W H, et al. Ultrathin nanotube/nanowire electrodes by spin–spray layer-by-layer assembly: a concept for transparent energy storage[J]. ACS Nano, 2015,9:10005−10017. doi: 10.1021/acsnano.5b03578 [47] Gu S C, Wang H L, Wu C, et al. Confirming reversible Al3+ storage mechanism through intercalation of Al3+ into V2O5 nanowires in a rechargeable aluminum battery[J]. Energy Storage Mater, 2017,6:9−17. doi: 10.1016/j.ensm.2016.09.001 [48] Tepavcevic S, Liu Y, Zhou D, et al. Nanostructured layered cathode for rechargeable Mg-ion batteries[J]. ACS Nano, 2015,9:8194−8205. doi: 10.1021/acsnano.5b02450 [49] Moretti A, Passerini S. Bilayered nanostructured V2O5·nH2O for metal batteries[J]. Adv Energy Mater, 2016,6:1600868. doi: 10.1002/aenm.201600868 [50] Diem A M, Fenk B, Bill J, et al. Binder-free V2O5 cathode for high energy density rechargeable aluminum-ion batteries[J]. Nanomaterials, 2020,10:247. doi: 10.3390/nano10020247 [51] Chen Z, Augustyn V, Wen J, et al. High-performance supercapacitors based on intertwined CNT/V2O5 nanowire nanocomposites[J]. Adv Mater, 2011,23:791−795. doi: 10.1002/adma.201003658 [52] Chen Z, Augustyn V, Jia X, et al. High-performance sodium-ion pseudocapacitors based on hierarchically porous nanowire composites[J]. ACS Nano, 2012,6:4319−4327. doi: 10.1021/nn300920e 期刊类型引用(0)
其他类型引用(1)
-






 下载:
下载:

 下载:
下载: