Effect of potassium hydroxide on preparation of rutile TiO2
-
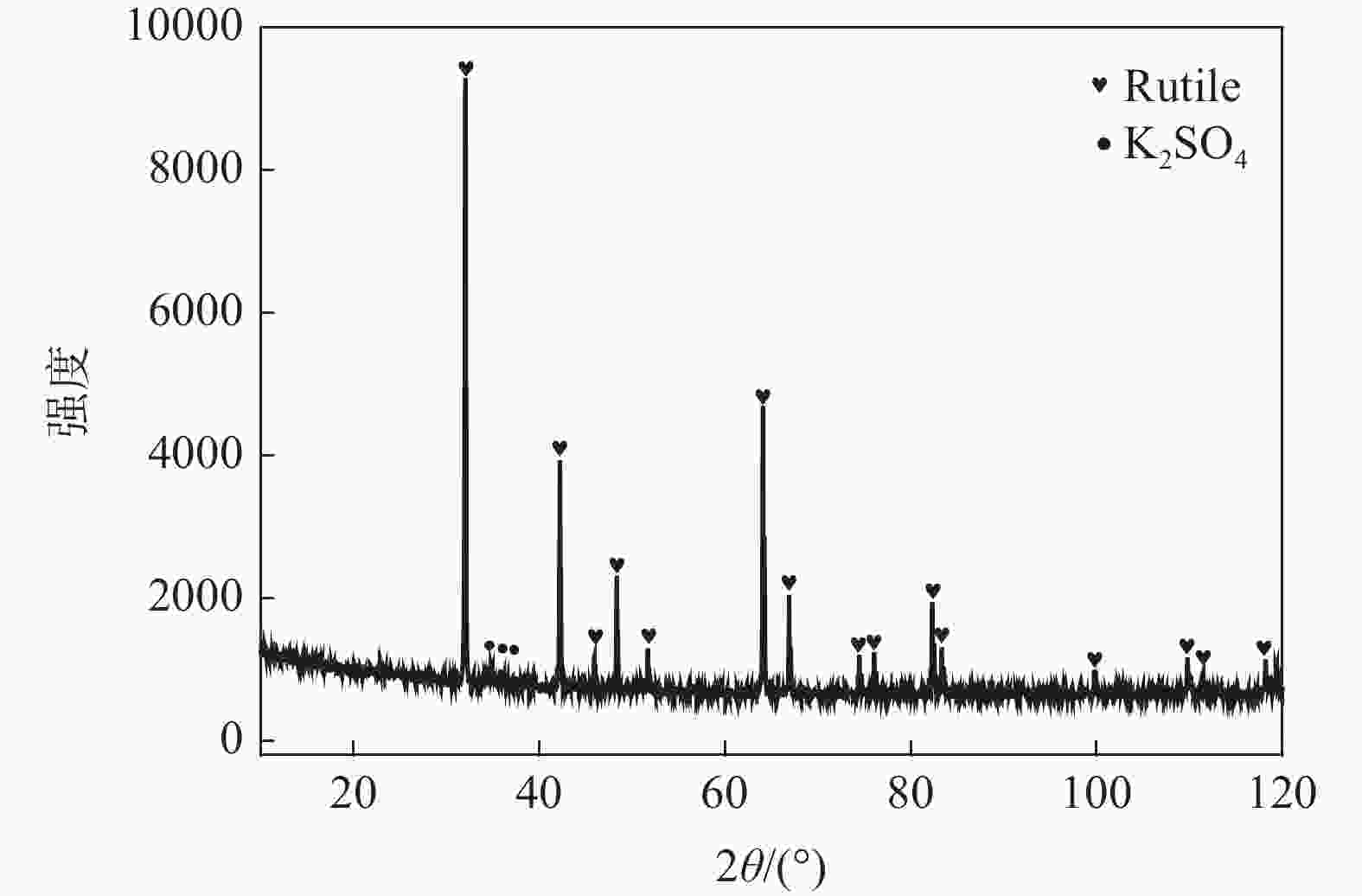
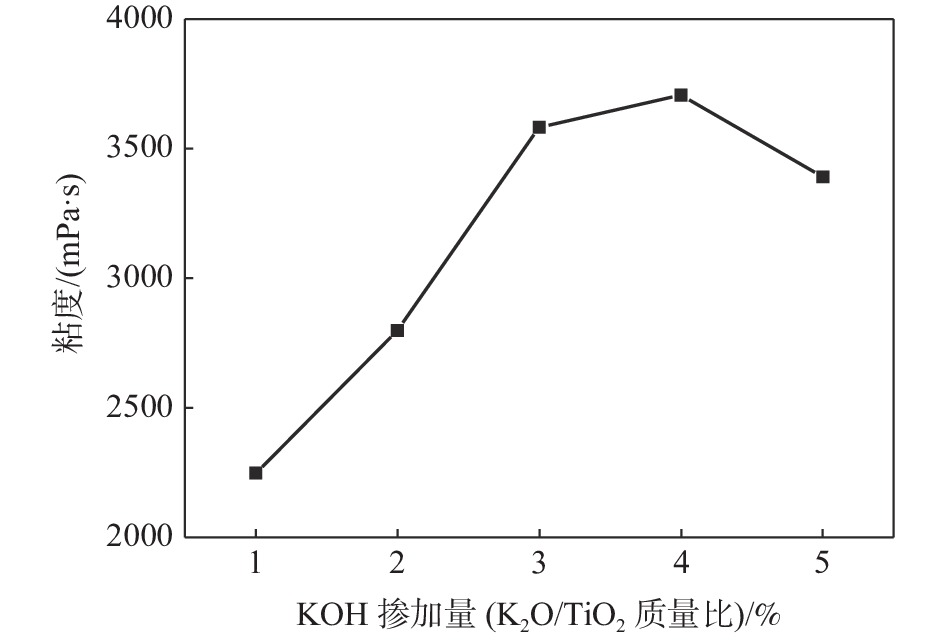
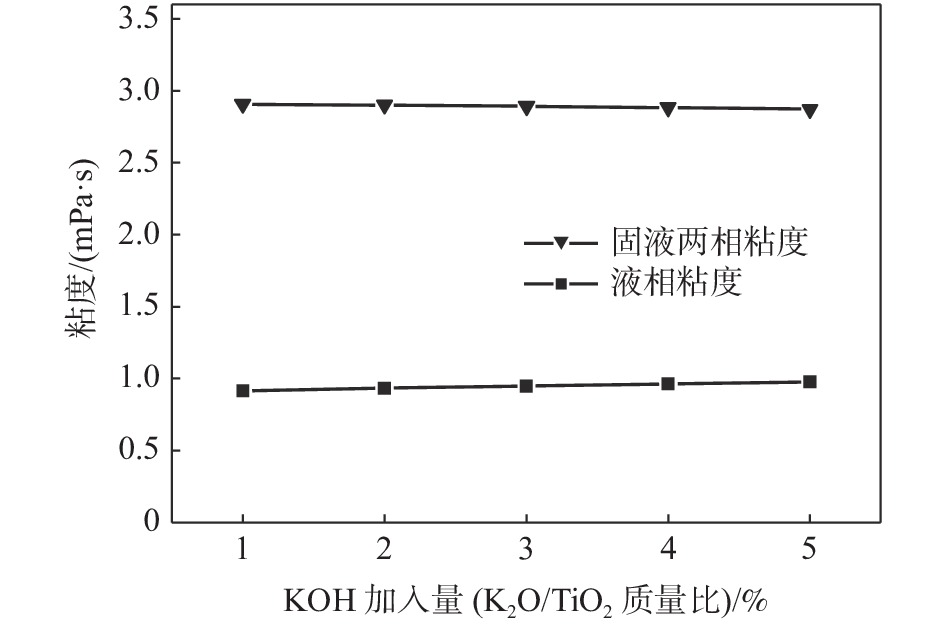
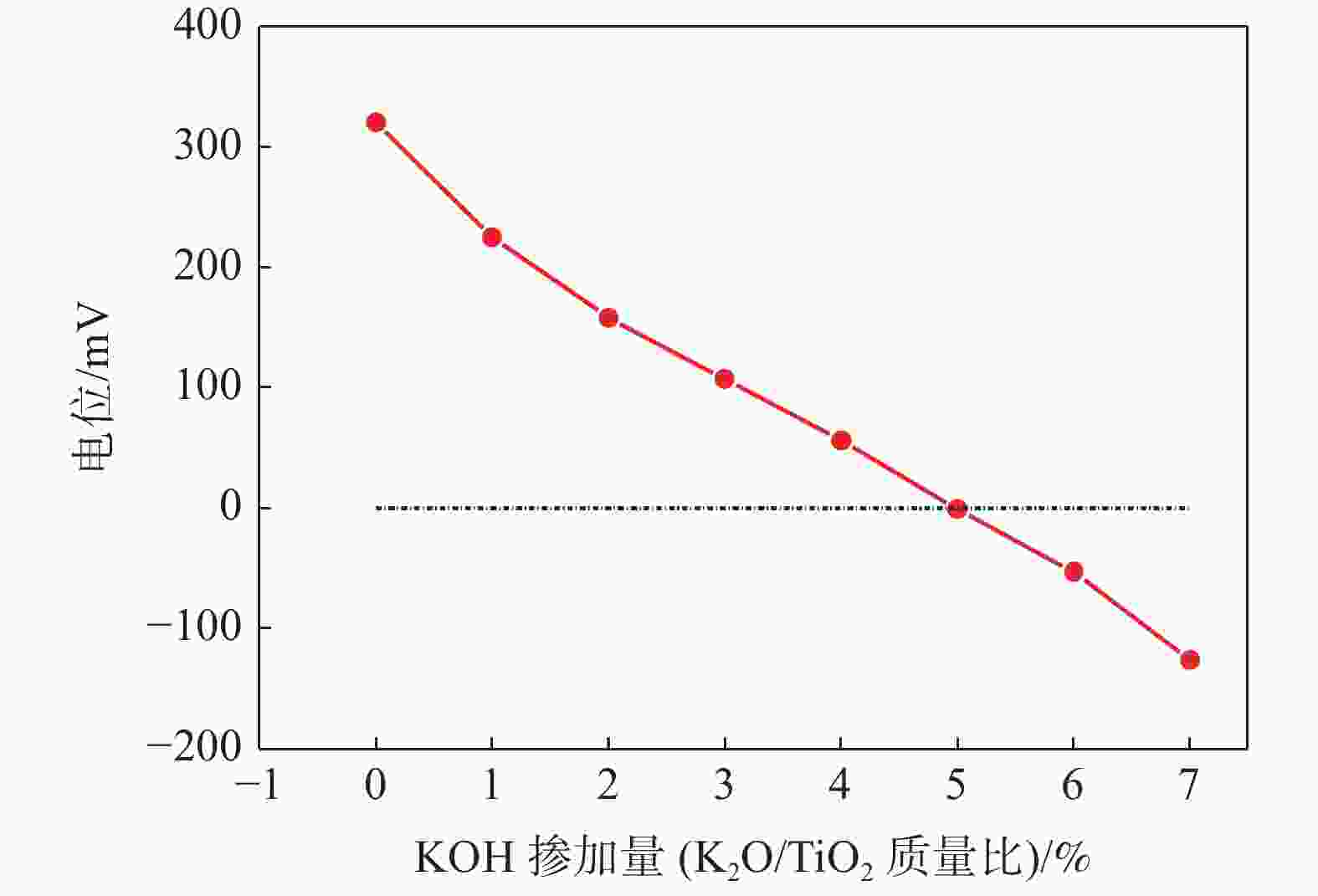
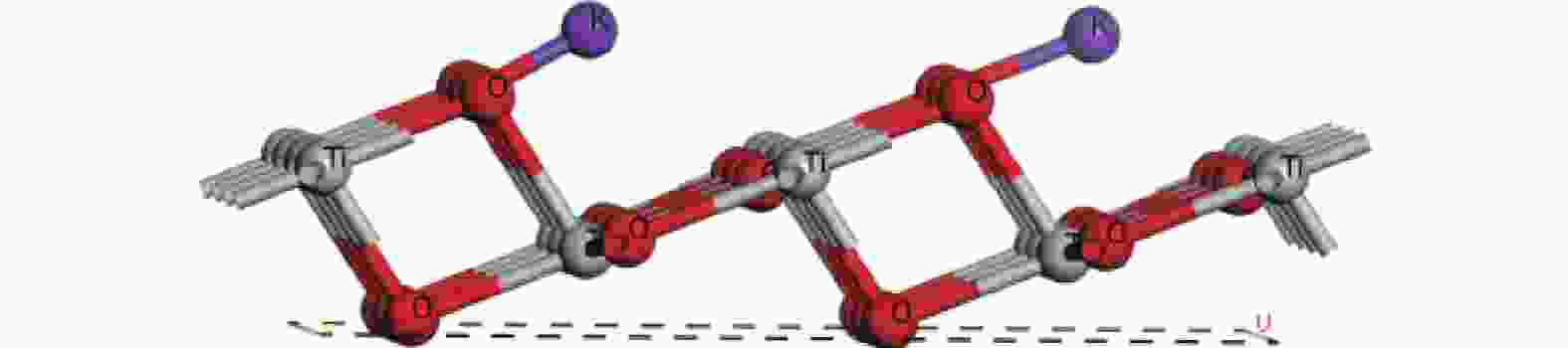
摘要: 向水合二氧化钛中添加一定量的氢氧化钾,研究从水合二氧化钛转变至金红石型二氧化钛过程中,钾在体系中的作用机理,钾对水合二氧化钛浆料的粒度和粘度、金红石型二氧化钛长径比和粒径分布的作用。采用拉曼光谱仪、马尔文粒度仪、XRD和电镜对样品进行了表征。结果表明:氢氧化钾加入水合二氧化钛体系中与残留的硫酸反应,生成了硫酸钾,在从水合二氧化钛至金红石型二氧化钛转变过程中起作用。氢氧化钾中的OH−会夺取水合二氧化钛中的H+,引起电位的变化,从而致使粘度先增后降。水合二氧化钛经过煅烧先转化成锐钛型 TiO2,然后转化成金红石型 TiO2,大量“K+”吸附在锐钛型 TiO2米勒面101面上,阻碍了二氧化钛表面的Ti-O构晶离子通过表面迁移,从而影响金红石型二氧化钛颗粒长径比和粒径分布。Abstract: A certain amount of potassium hydroxide was added to the hydrated titanium dioxide to study the mechanism of potassium in the system during the transition from hydrated titanium dioxide to rutile titanium dioxide, and the effect of potassium on the particle size and viscosity of hydrated titanium dioxide slurry, the aspect ratio and particle size distribution of rutile titanium dioxide. The samples were characterized by Raman spectrometer, Malvin particle size analyzer, XRD and SEM. Results showed that potassium hydroxide reacted with residual sulfuric acid in hydrated titanium dioxide system to produce potassium sulfate, which played a role in the transition from hydrated titanium dioxide to rutile titanium dioxide. The OH− in potassium hydroxide will seize the H+ in hydrated titanium dioxide, causing the change of potential, resulting in the viscosity first increasing and then decreasing. The hydrated TiO2 was transformed into anatase TiO2 after calcination, and then into rutile TiO2, a large amount of K+ adsorbed on the (101) facets of anatase TiO2 hindered the migration of Ti–O crystal ions through the surface, thus affecting the aspect ratio and particle size distribution of rutile TiO2 particles.
-
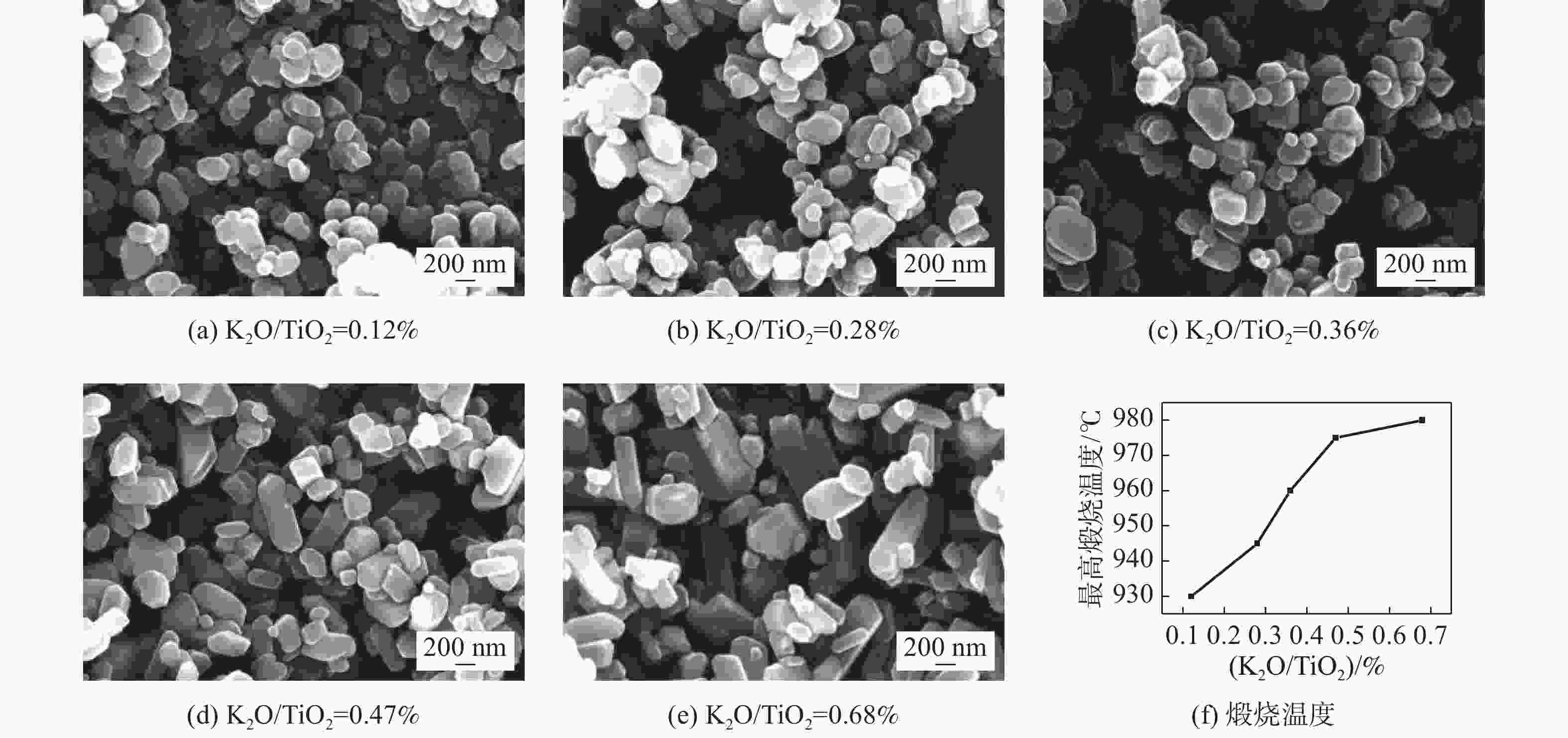
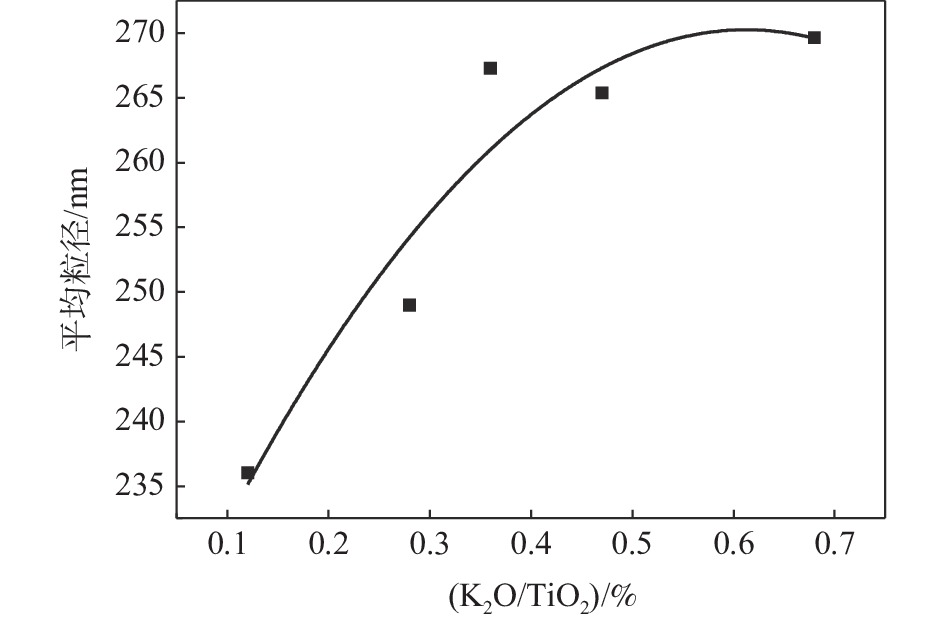
表 1 样品颗粒长径比分布
Table 1. Length-diameter ratio distribution of samples
序号 (K2O/TiO2 )/ % 长径比分布 平均长径比 1 0.12 1.0~2.2 1.35 2 0.28 1.0~3.4 1.42 3 0.36 1.0~4.0 1.48 4 0.47 1.0~4.4 1.62 5 0.68 1.0~4.6 1.73 表 2 样品粒径分布
Table 2. Size distribution of samples
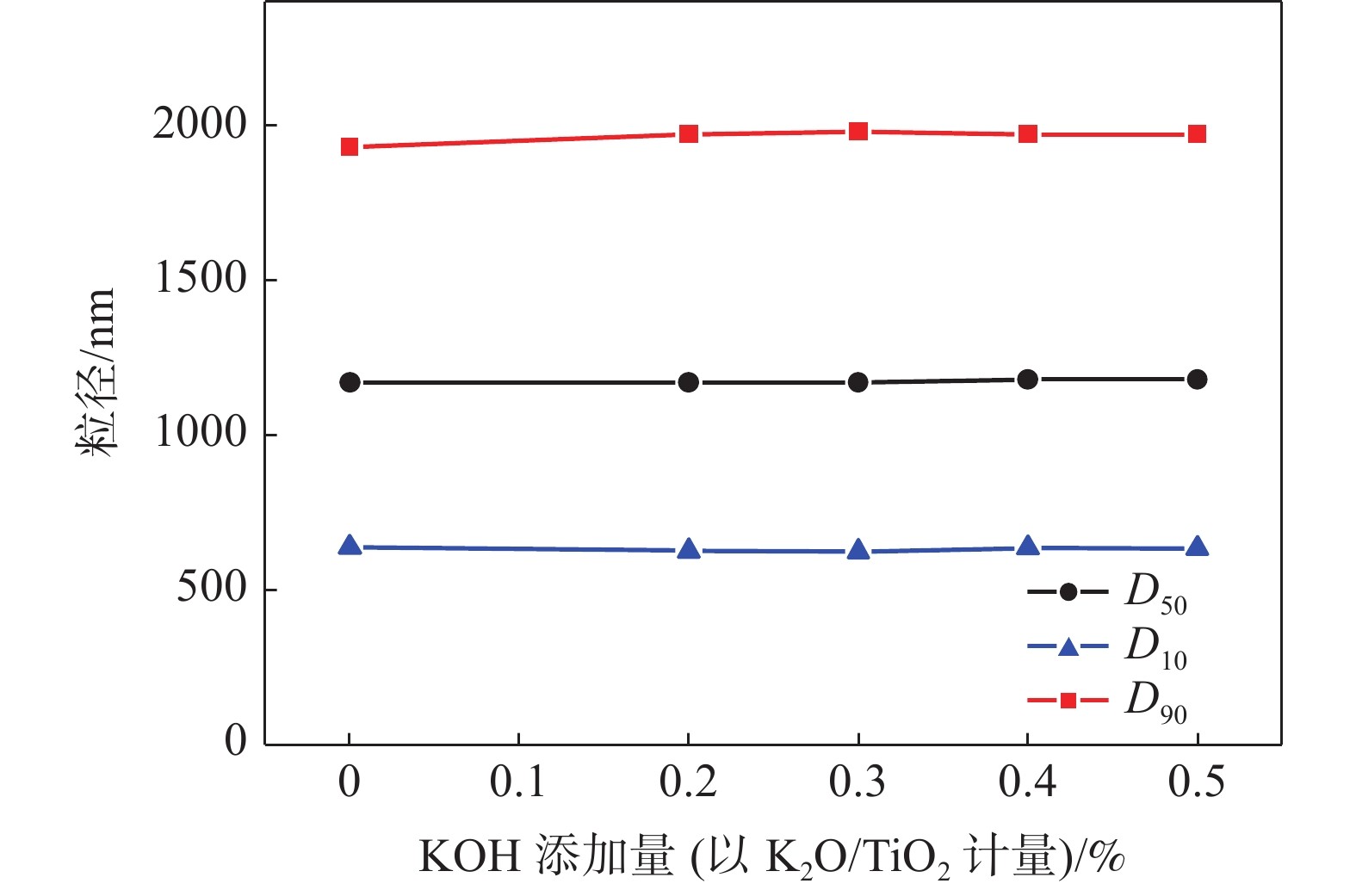
(K2O/TiO2 )/ % 粒度(nm)占比/% <150 150~200 200~250 250~300 300~350 350~400 400~450 450~500 500~550 >550 0.12 7.53 18.28 20.43 17.2 21.51 8.6 2.15 0 2.15 2.15 0.28 7.2 20.8 22.4 11.2 13.6 7.2 7.2 3.2 0.8 6.4 0.36 5.41 10.81 13.51 31.08 10.81 6.76 6.76 8.11 1.35 5.4 0.47 9 9 17 22 16 5 2 5 4 11 0.68 16.83 21.29 19.31 11.39 8.91 8.42 4.46 1.98 0.99 6.44 -
[1] Ma Weiping. Effect of potassium hydroxide on calcination of metatitanic acid[J]. Iron Steel Vanadium Titanium, 2019,40(4):35. (马维平. 偏钛酸煅烧过程中氢氧化钾作用研究[J]. 钢铁钒钛, 2019,40(4):35. doi: 10.7513/j.issn.1004-7638.2019.04.007 [2] 裴润, 沈宗琦, 吴永宝, 等. 硫酸法钛白生产[M]. 北京: 化学工业出版社, 1982: 157.Pei Run, Shen Zongqi, Wu Yongbao, et al. Production of titanium dioxide by sulfuric acid process[M]. Beijing: Chemical Industry Press, 1982: 157. [3] 唐振宁. 钛白粉的生产与环境治理[M]. 北京: 化学工业出版社, 2000: 111.Tang Zhenning. Production and environmental control of titanium dioxide[M]. Beijing: Chemical Industry Press, 2000: 111. [4] 吴德荣, 汪镇安, 王江义, 等. 化工工艺设计手册(上册)[M]. 北京: 化学工业出版社, 2009: 1242.Wu Derong, Wang Zhen, an, Wang Jiangyi, et al. Chemical process design manual ( Volume I )[M]. Beijing: Chemical Industry Press, 2009: 1242. [5] Bamforth A W. Industrial crystallization[M]. London: Leonard Hiu, 1965: 142-163. [6] 陆佩文. 无机材料科学基础[M]. 武汉: 武汉工业大学出版社, 1996: 42.Lu Peiwen. Fundamentals of inorganic materials science[M]. Wuhan: Wuhan University of Technology Press, 1996: 42. -




 下载:
下载: