Thermodynamic analysis of the effect of oxygenation on the low-temperature chlorination selectivity of carbonized slag
-
摘要: 利用Factsage热力学软件及数据库,计算分析了引入氧气对碳化钛渣中TiC和钙镁氧化物氯化的影响,以及加氧选择性强化碳化渣中TiC氯化反应的同时减少或抑制钙镁氧化物氯化反应的调控方案。结果表明,加氧低温氯化可促进渣中TiC的氯化反应进程,提高TiC的氯化率;当低温氯化反应温度为500 ℃时,在Cl2/TiC摩尔比为1.80~3.50范围内,MgO不发生氯化反应,氧气的引入可以使CaO氯化反应受到抑制,降低CaO氯化率;氯氧气体摩尔比为4.00:1.00,氯化反应使碳化渣中TiC质量分数减小至4.02%时,可将氯化气体切换为氯气与氮气的混合气体,进一步氯化渣中残留的TiC直至2.50%以下;此加氧分步氯化与不加氧直接氯化相比,氯气总消耗量可减少25.69%,CaO总氯化率可减少37.74%。Abstract: Based on the Factsage thermodynamic software and database, the effects of introducing oxygen on the TiC and calcium magnesium oxide chlorination in titanium carbide slag are analyzed, and the regulatory scheme of adding oxygen to selectively enhance the chlorination reaction of TiC in the slag while reducing or inhibiting the chlorination reaction of calcium and magnesium oxides are studied. The results show that the oxygenated low-temperature chlorination can promote the chlorination process and increase the chlorination rate of TiC in the slag. When the low-temperature chlorination reaction temperature is 500 ℃ and the molar ratio of Cl2/TiC is 1.80~3.50, MgO does not chlorinate, and the introduction of oxygen can inhibit the CaO chlorination reaction and reduce the CaO chlorination rate. When the molar ratio of chlorine-oxygen gas is 4.00:1.00, and the mass fraction of TiC with chlorination reaction in carbonization slag is reduced to 4.02%, the chlorinated gas can be switched to a mixture of chlorine gas and nitrogen gas to further chlorinate the residual TiC in the slag to less than 2.50%. Compared with direct chlorination without adding oxygen, the total chlorine consumption can be reduced by 25.69% and the total chlorination rate of CaO can be reduced by 37.74%.
-
0. 引言
TiCl4作为生产钛白粉和海绵钛的重要原料,其各种制备工艺已得到广泛关注和研究[1-3]。高温碳化高钛高炉渣(即碳化钛渣,简称“碳化渣”)-低温(500 ℃左右)选择性氯化工艺相较于熔盐氯化和沸腾氯化工艺具有氯化温度低、处理能力强,对原料中钙镁氧化物含量无严格限制等优点,已成为目前最具工业应用前景的工艺技术[4-5]。然而,由于仍然存在部分钙镁杂质氧化物的氯化,生成不易挥发的高沸点物质CaCl2、MgCl2,这两种氯化物在氯化炉内还可形成共熔温度为617 ℃的固溶体mCaCl2·nMgCl2。虽然氯化温度只有500 ℃左右,但TiC氯化反应过程中放出大量的热量会导致炉内局部温度升高达到或超过固溶体mCaCl2·nMgCl2的熔化温度,从而形成液相,引起颗粒物料粘结甚至影响碳化渣流态化稳定性。如何提高碳化渣中钛的氯化率同时拟制或减少钙镁杂质氧化物的氯化成为亟待探索解决的重要问题。
彭毅[6]从热力学角度分析认为,抑制CaO、MgO等杂质氧化物氯化的关键在于阻止氧化物的加碳氯化。如果在反应体系中引入适量的氧气,使生成的碳燃烧生成CO2,改变TiC氯化的吉布斯自由能和平衡常数,可以有效地抑制钙镁等杂质氧化物加碳氯化反应,从而达到选择性氯化提钛的目的。刘晓华[7]进行了氧气携带氯气氯化碳化高炉渣初步试验研究,结果表明有氧氯化反应相较于无氧氯化反应的氯化残渣粘结程度减弱,表明氧气的引入对于缓解物料粘结有一定作用等。但目前尚未见关于引入氧气对碳化渣低温氯化提钛与抑制钙镁杂质氧化物氯化反应影响作用的研究报道。与此相关的其它研究发现,Taki[8]等人利用氯气和氧气的混合气体在1223 K温度下进行了从磷矿中选择性提取铀的研究,结果表明,引入氧气可以降低铝、磷和硅氯化率,同时铀的氯化率达到90%以上;M Hiroyuki[9-10]等人研究了从电弧炉粉尘中回收重金属的工艺,粉尘中主要含铁、铅和锌的氧化物,在高氯高氧分压下,铁的稳定相为氧化物,而锌和铅的稳定相为氯化物,表明通过引入含有氯气和氧气的混合气体可以对重金属进行选择性氯化过程进行回收;Jungshin K[11]等人进行了低品位钛矿在高氧化学势下的选择性氯化制备高品位TiO2同时去除矿石中的铁的基础研究,Fe-O-Cl和Ti-O-Cl体系的化学势图表明,当Cl2和O2分压位于一个合适的区域时铁氧化物将被氯化形成FeClx(l,g)[x=2,3],而钛氧化物将不被氯化而仍以TiO2(s)形式保留下来。试验结果表明在一定条件下铁以氯化铁(FeClx[x=2,3])形式被选择性去除,并得到了98%的TiO2。说明加氧有协同或拟制体系中不同物质氯化反应进程的作用,以实现选择性氯化提取分离方法的可行性。
笔者采用Factsage热力学计算软件FactPS和Ftoxid数据库对加氧氯化碳化钛渣的反应进行分析,探索不同氧氯比对碳化钛渣中各物质氯化反应的影响及选择性控制条件,并结合实际碳化渣组分含量,计算分析合适的氧气引入量、氧氯气体占比及其与碳化渣中TiC、CaO、MgO组分含量变化的调控关联关系。
1. 热力学计算方法及参数设置
热力学性质和相平衡用Gibbs自由能最小算法计算,在等温、等压的多元体系中,平衡条件为体系的吉布斯自由能G最小,即:
$$ {{G}}_{\text{min}}={G}\text={\sum }_{{i}}{{n}}_{{i}}{{\mu}}_{{i}}={\sum }_{{i}}{{n}}_{{j}}\left({{\mu}}_{{i}}^{{0}}+{{\rm{R}}T\ln}{\text{∂}}_{{i}}\right) $$ (1) 式中,G为Gibbs自由能,J;i为体系中组元;j为组元i中元素;ni为组元i物质的量,mol;nj为元素j物质的量,mol;μi为组元i的化学势,J/mol;μi0为组元i的标准化学势,J/mol;∂ i为组元i在温度T下的原子数,mol;R为理想气体常数,J/(K·mol);T为体系温度,K。
笔者应用热力学计算软件Factsage8.1,选择配套的FactPS和Ftoxid数据库,利用Reaction、Equilib和Phase Diagram模块,以攀钢高炉渣碳化后的碳化渣为研究对象,其主要化学成分及含量如表1所示。以低温氯化温度500 ℃为例,计算分析引入氧气量多少对碳化渣中主要成分如TiC、CaO和MgO氯化反应的影响与调控条件。体系压力均设为1.00 atm(0.1 MPa)。
表 1 碳化渣的主要成分及含量Table 1. Main components and content of carbonized slag成分 质量分数/% 摩尔数/mol* 摩尔比* TiC 13.80 0.23 1.00 CaO 26.57 0.47 2.04 MgO 8.09 0.20 0.87 SiO2 25.25 0.42 1.83 Al2O3 14.23 0.14 0.61 MnO 0.55 0.01 0.04 Fe 1.00 0.02 0.09 V2O5 0.24 0.01 0.04 TiO2 3.60 0.05 0.22 FeO 2.00 0.03 0.13 C 4.00 0.33 1.43 其它 0.67 *:以100 g碳化渣计的摩尔数和以TiC=1.00 mol为基折算的摩尔比。 2. 结果与讨论
2.1 加氧氯化反应趋势的热力学分析
为了预测碳化渣低温氯化反应中引入一定含量的氧气后对体系中各组分氯化反应的影响,利用Reaction和Phase Diagram模块,分别计算了碳化渣低温氯化反应过程中,体系中可能存在的反应在不同温度下(100~1000 ℃)的吉布斯自由能,以及提钛主反应TiC-Cl2-O2体系在500 ℃条件下的平衡物相与氯气和氧气分压的关系。
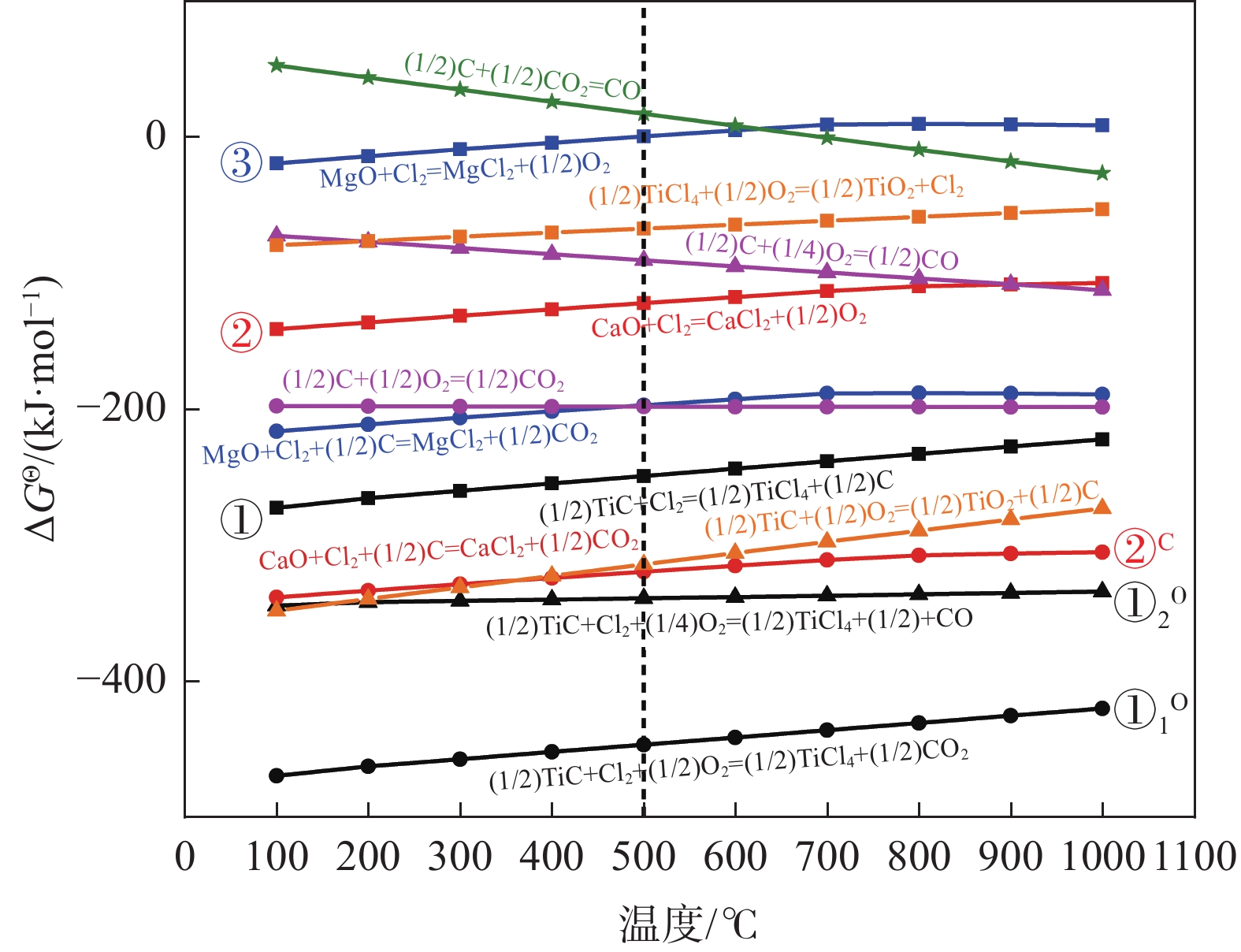
图1为各反应式中Cl2和C分别以1.00 mol和0.50 mol为基准的吉布斯自由能。由图1可见,当氯化温度在500 ℃(图中虚线所示)左右,未引入氧气时,TiC、CaO和MgO均可发生氯化反应(图1中①、②、③所示),且CaO加碳氯化反应(图1中② C所示)的吉布斯自由能比TiC直接氯化反应(图1中①)的吉布斯自由能更小,表明其氯化趋势在低温氯化提钛炉内较为明显;当引入氧气后,TiC可发生加氧氯化反应(图1中①1O和①2O所示),TiC中的C直接与O结合形成CO或CO2气体,其反应吉布斯自由能明显下降,表明氧气的引入有促进选择性氯化TiC反应的趋势,即碳化渣中的TiC在氧气存在条件下的氯化趋势更明显。
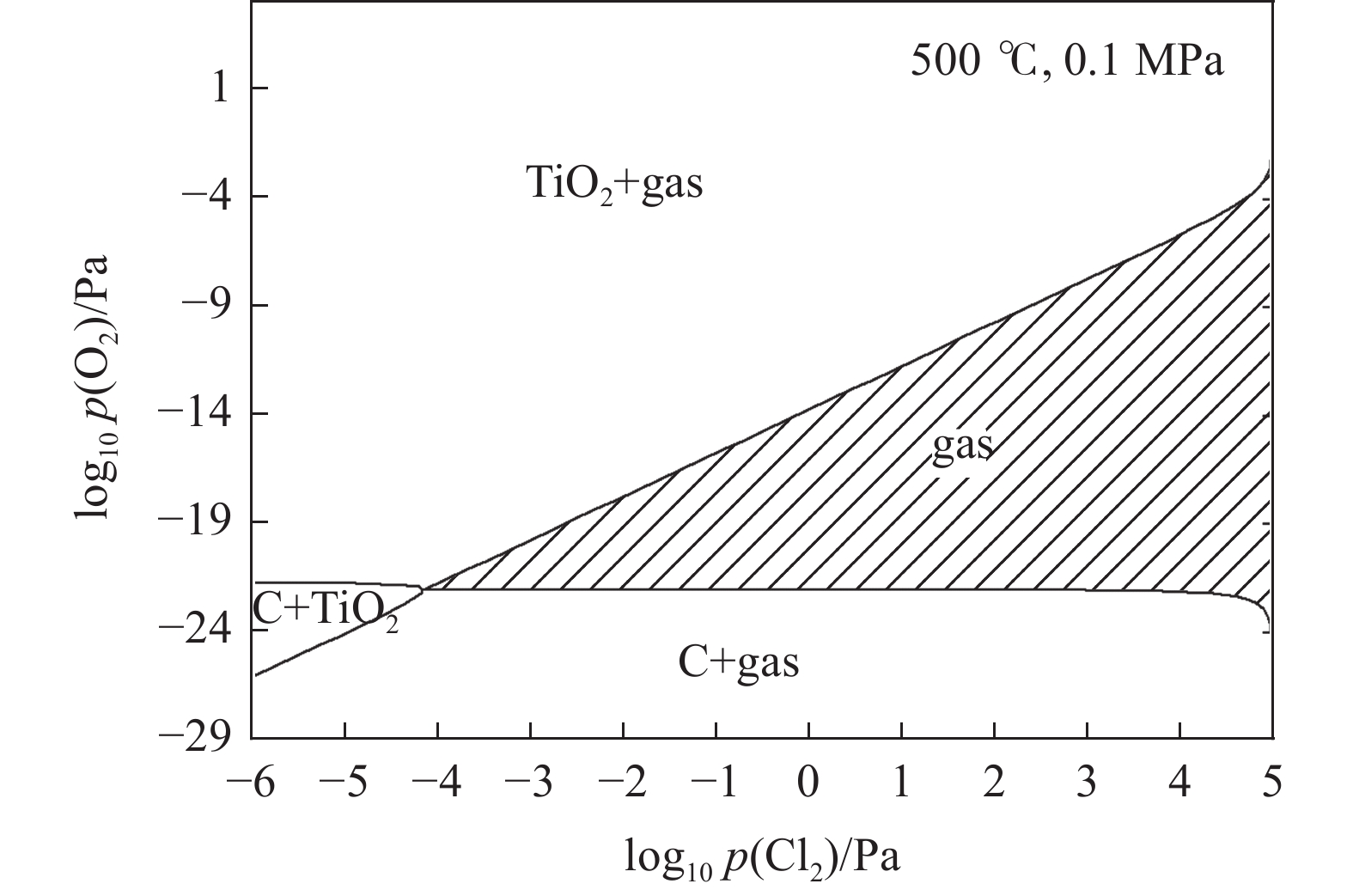
以氯化温度为500 ℃ 为例的提钛主反应TiC-Cl2-O2体系,如图2阴影区域所示,在高氯低氧分压条件下,反应产物可全部为稳定的气体gas,主要物质(TiCl4、Cl2、CO和CO2),这表明在碳化渣氯化过程中引入氧气可阻止单质碳的析出,适当调控有减缓甚至避免钙镁等杂质氧化物因有碳协同的氯化反应发生。
2.2 加氧氯化对TiC、CaO和MgO氯化反应影响的热力学分析
由表1可得,碳化渣中TiC与CaO、MgO的摩尔比分别为1.00∶2.04和1.00∶0.87。因此,设定TiC-CaO-MgO-Cl2-O2体系中TiC、CaO、MgO的摩尔数分别为1.00、2.04、0.87 mol。采用Equilib模块计算了Cl2、O2含量变化对不同反应体系中钙镁氧化物氯化反应的影响。
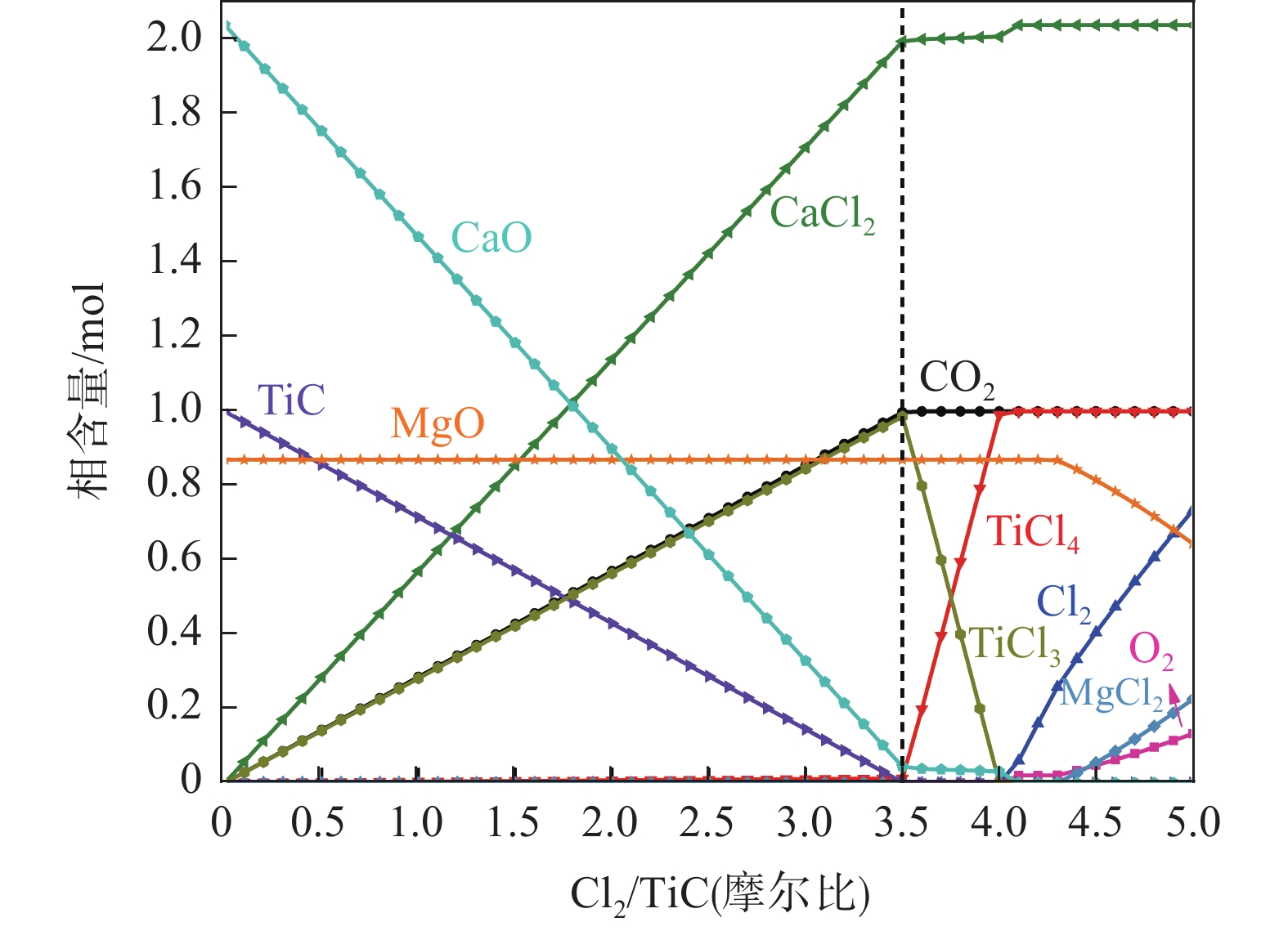
图3为TiC-2.04CaO-0.87MgO-nCl2体系下平衡物相含量随Cl2含量变化规律(Cl2含量变化以Cl2/TiC摩尔比计)。由图3可见,随着Cl2含量逐渐增加,体系中TiC和CaO均被逐渐氯化,其中含钛物相转变路径为TiC→TiCl3→TiCl4;在Cl2含量约为3.50 mol时TiC含量减少至零,CaO含量减少至0.04 mol;而体系中的MgO在TiC和CaO未反应完全之前并未被氯化,其含量始终维持在初始值0.87 mol,表明TiC和CaO优先于MgO的氯化。为此,在后续计算中设定Cl2/TiC摩尔比值最大为3.50。
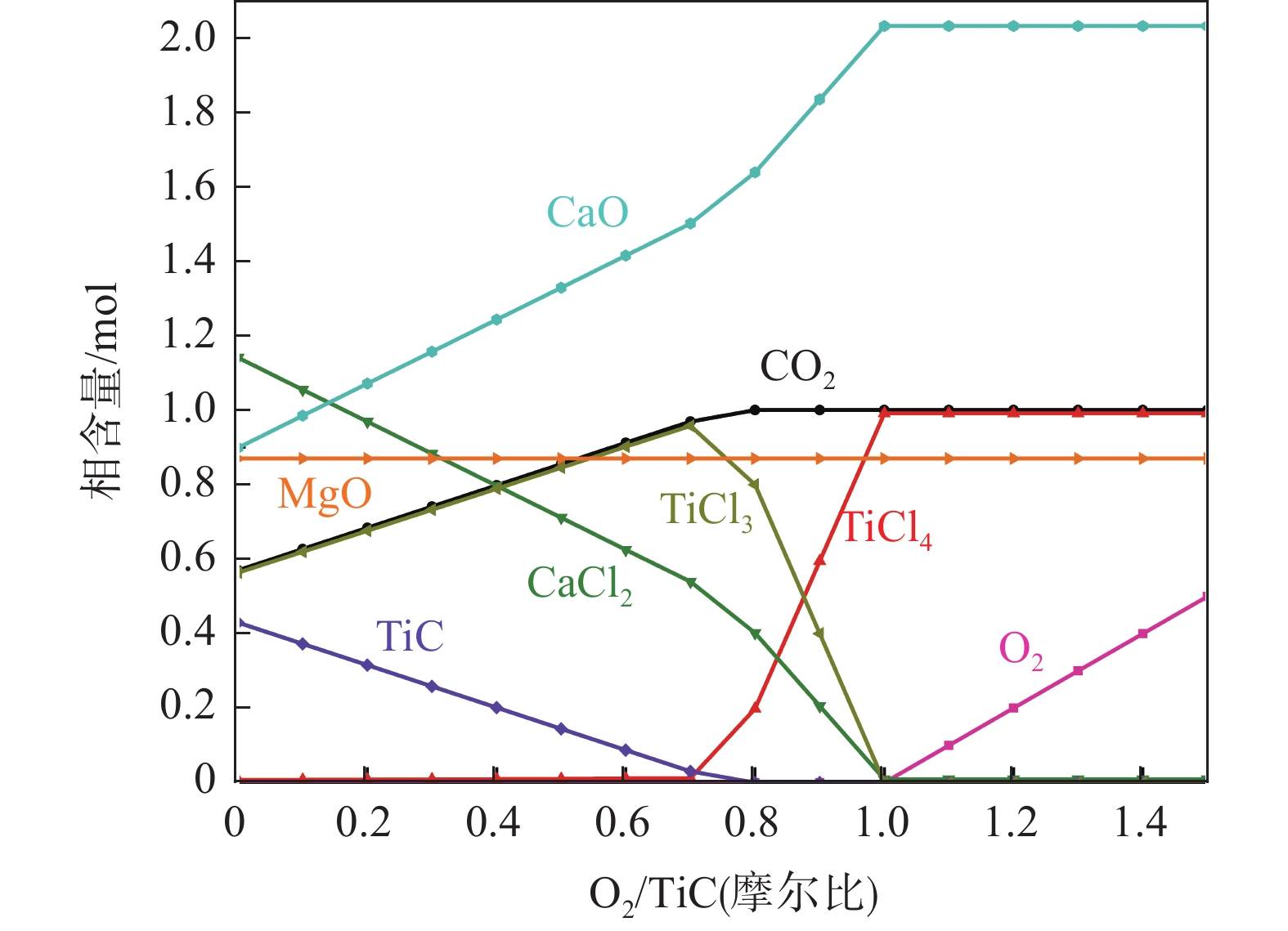
图4为TiC-2.04CaO-0.87MgO-2.00 Cl2-mO2体系平衡物相含量随O2含量变化规律(O2含量变化以O2/TiC摩尔比计)。
由图4可见,在TiC-2.04CaO-0.87MgO-2.00 Cl2-mO2体系中,无论是否引入氧气,MgO均未发生氯化反应,其含量一直维持0.87 mol不变,因此,在后续文中不再阐述MgO随氯气含量和氧气含量的变化;未引入氧气时,TiC和CaO均部分被氯化,而在体系中引入氧气后TiC氯化反应不仅可以得到促进,而且在达到最优O2/TiC摩尔比之前随着引入氧气含量逐渐增多,产生的CaCl2含量逐渐减少,表明CaO的氯化反应在引入氧气后受到抑制。
表2为不同Cl2/TiC摩尔比(以Cl2含量计为1.80~3.50)体系引入氧气前后TiC和CaO氯化率变化规律,其中,引入氧气后TiC和CaO的氯化率在最优O2/TiC摩尔比(定义为TiC完全氯化且CaO有最小氯化率)下得到,TiC、CaO氯化率计算方法如下:
表 2 TiC-2.04CaO-0.87MgO-nCl2-mO2反应体系中各物相氯化率随O2含量变化规律Table 2. The variation of chlorination rates of various phases with O2 contents in the TiC-2.04CaO-0.87MgO-nCl2-mO2 reaction systemCl2含量n TiC氯化率/% CaO氯化率/% 最优O2/TiC 摩尔比 Cl2/O2摩尔比 未引入氧气 引入氧气 氯化率变化情况 未引入氧气 引入氧气 氯化率变化情况 1.80 51.35 100.00 +48.65 50.33 0.00 −50.33 1.10 1.64 1.90 54.20 100.00 +45.80 53.12 0.00 −53.12 1.10 1.73 2.00 57.06 100.00 +42.94 55.88 0.49 −55.39 1.00 2.00 2.10 59.91 100.00 +40.09 58.82 5.39 −53.43 1.00 2.10 2.20 62.76 100.00 +37.24 61.27 10.29 −50.98 0.90 2.44 2.30 65.62 100.00 +34.38 64.22 15.20 −49.02 0.90 2.56 2.40 68.47 100.00 +31.53 67.16 20.10 −47.06 0.80 3.00 2.50 71.32 100.00 +28.68 70.10 25.00 −45.10 0.80 3.13 2.70 77.03 100.00 +22.97 75.49 34.80 −40.69 0.70 3.86 2.90 82.74 100.00 +17.26 80.88 44.61 −36.27 0.60 4.83 3.10 88.44 100.00 +11.56 86.76 54.41 −32.35 0.50 6.20 3.30 94.15 100.00 +5.85 92.16 64.22 −27.94 0.40 8.25 3.50 100.00 100.00 +0.00 98.04 74.02 −24.02 0.30 11.67 $$ {{C}}_{\text{1}}\text=\frac{{\Delta }{{M}}_{\text{1}}}{{{M}}_{\text{1}}}{\times100\%} $$ (2) $$ {{C}}_{\text{2}}\text=\frac{{\Delta }{{M}}_{\text{2}}}{{{M}}_{\text{2}}}{\times 100\%} $$ (3) 式中, M1和M2分别代表TiC和CaO的初始摩尔量;ΔM1和ΔM2分别代表TiC和CaO 被氯化消耗的摩尔量;C1和C2 分别代表TiC和CaO的氯化率。
由表2可见,在不同Cl2含量下,未引入氧气时,随着Cl2含量增加即Cl2/TiC摩尔比逐渐增大,TiC氯化率逐渐升高,但同时体系中CaO的氯化率也升高;而引入合适量的氧气后,CaO氯化率减小了24.02%~55.39%。随着Cl2/TiC摩尔比从1.80逐渐增加到3.50,完全氯化TiC所需的最优O2含量也从1.10减小到0.30,主要是因为在氧气存在下TiC中的C不以C单质形式析出,而是转变成CO或CO2逸出氯化炉内,随着体系中Cl2含量增加导致CaO被氯化产生氧气增多,达到补充作用,造成O2/TiC摩尔比减小。因此,对于TiC-2.04CaO-0.87MgO-nCl2-mO2体系(n=1.80~3.50),当引入的氧气量达到最优时,Cl2/O2摩尔比为1.64~11.67,体系中TiC氯化率可达到100.00%,而CaO氯化率为0~74.02%。
2.3 加氧氯化分段式反应的气氛与物料转移的热力学分析
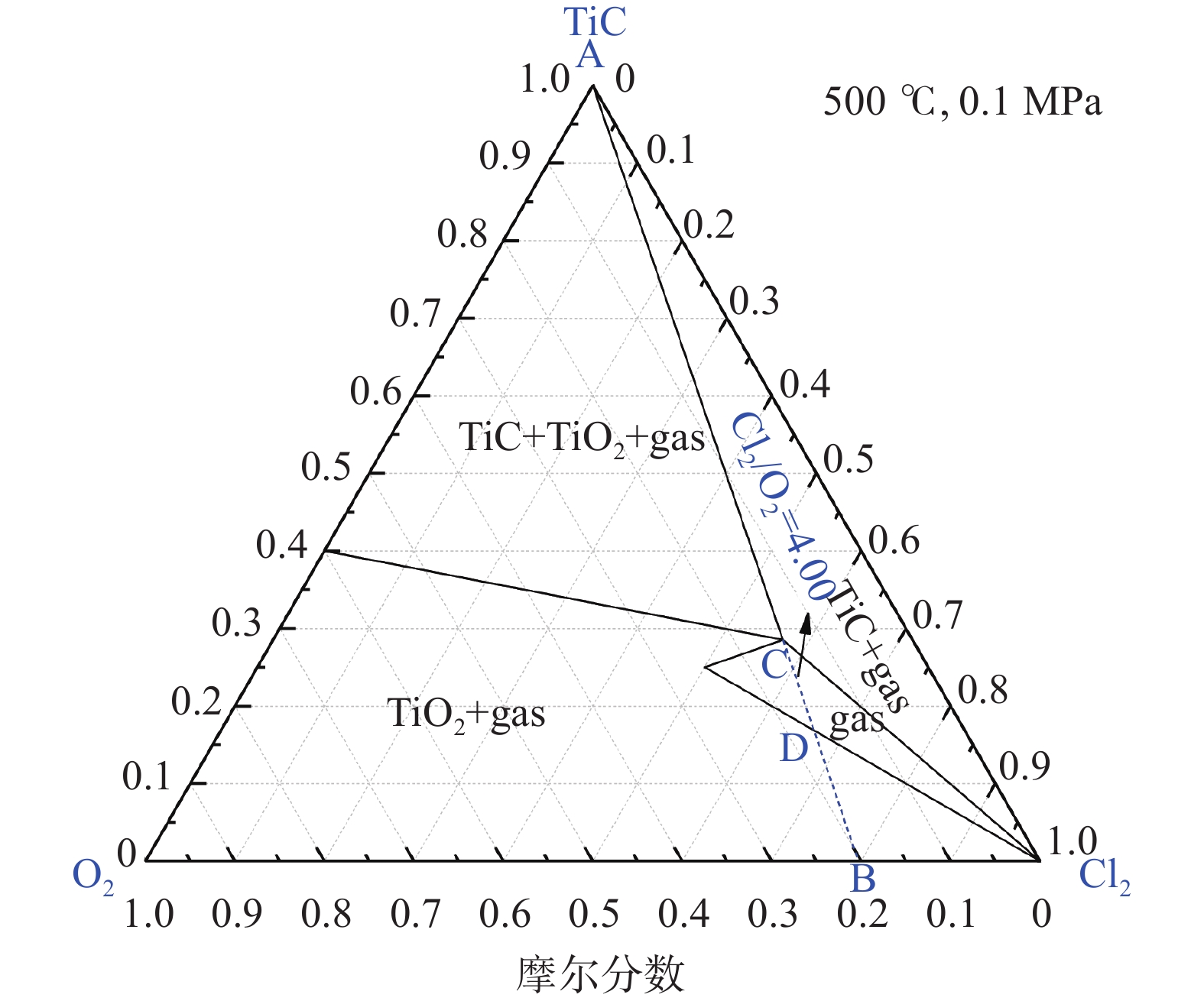
为提高钛的氯化提取率和降低氧化钙等杂质的氯化,有必要将碳化渣的低温氯化进行分段调节控制。为确定反应气体(氯气和氧气)组成及物料转移临界点,以低温氯化温度500 ℃为例,首先利用Phase Diagram模块计算了TiC-Cl2-O2体系的三元平衡相图,如图5所示,该相图共有四个不同的相区(TiC+TiO2+gas、TiC+gas、gas和TiO2+gas),其中gas相区的存在表明在合适的Cl2和O2比例下,反应产物可全部为气体(主要气体物质为TiCl4、Cl2、CO和CO2)。通过分析相图并结合考虑存在CaO时TiC-CaO-Cl2-O2体系含钛物相随Cl2和O2含量增加时的反应转变,综合考虑确定了提高TiC氯化率的同时减少或抑制氧化钙等杂质氧化物氯化的调控方案。
由图5可知,TiC+TiO2+gas相区与TiC+gas相区间存在分界线AB,即体系中Cl2/O2摩尔比值为4.00,也即是当Cl2/O2摩尔比值小于4.00时,在TiC氯化反应处于AB线的左侧相区,会有TiO2物相析出,反之,反应进程则在AB线的右侧相区内进行,在图中D点以上区域没有TiO2的产生。假设TiC总量为1.00 mol,在TiC-Cl2-O2相平衡体系中设定Cl2/O2摩尔比值为4.00(图5中AB线),此条件下TiC在被氯化减少过程中体系物相变化路径为:TiC→TiC+gas→gas→TiO2+gas。AB线与gas单相区的上边界和下边界分别相交于图5中C点和D点,C点处TiC含量为0.285 mol,D点处TiC含量为0.169 mol,表明当TiC量维持在此区间时,被氯化的TiC可全部转变成TiCl4,若进一步氯化减少TiC量则会有TiO2析出,不利于Ti的进一步提取。
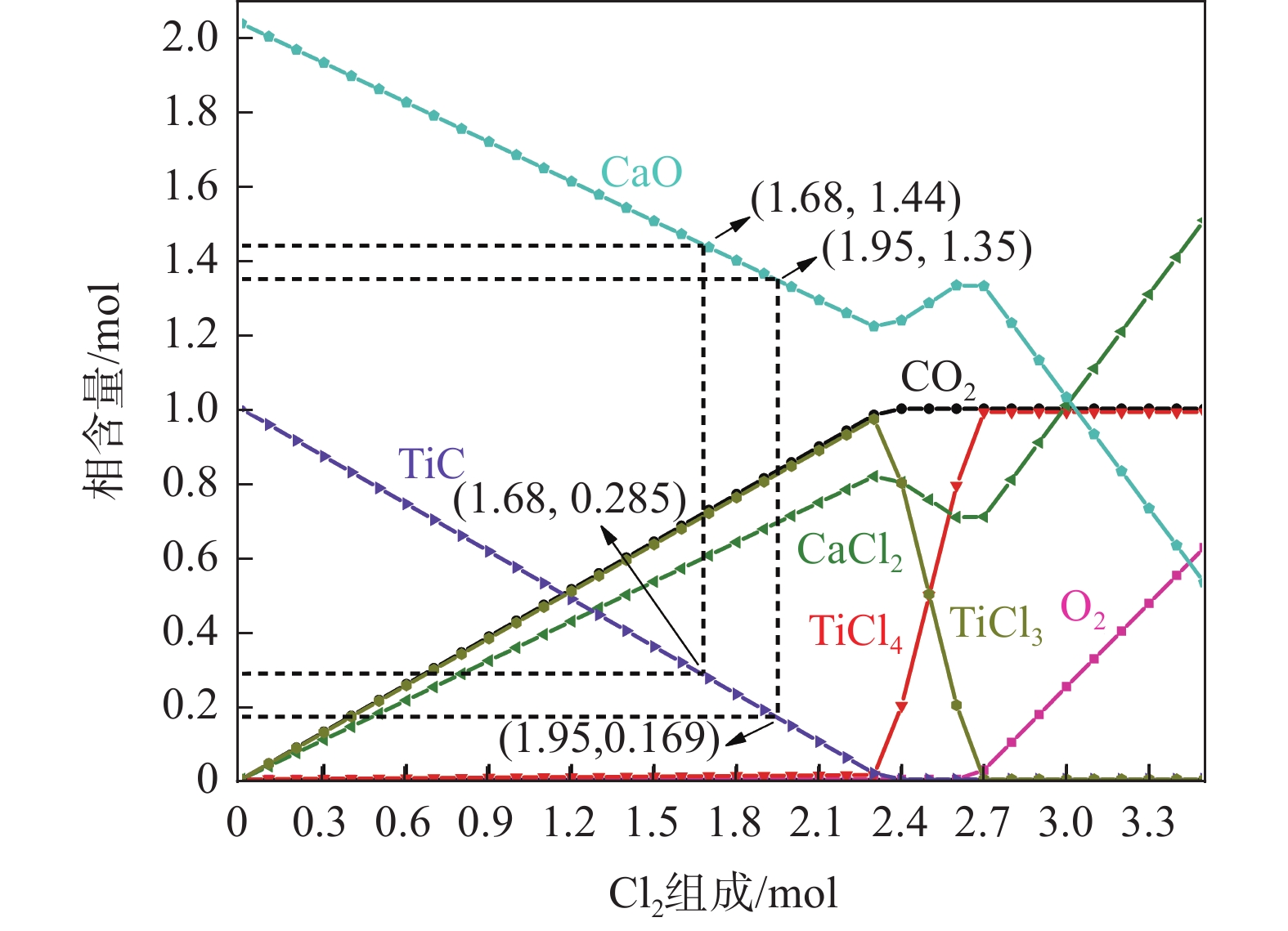
实际反应体系中存在可能被同时氯化的CaO,针对表1中所示的碳化渣成分,计算Cl2/O2摩尔比为4.00时的TiC-2.04 CaO-nCl2-0.25nO2体系平衡物相含量随Cl2和O2含量变化的规律,如图6所示。由图6可见,当TiC含量减少到0.285 mol时,CaO含量为1.44 mol,TiC和CaO的氯化率分别为71.50%和29.41%;而当TiC含量减少到0.169 mol时,CaO含量为1.35 mol,TiC和CaO的氯化率分别为83.10%和33.82%。
综上,为获得尽可能高的TiC氯化收得率提取TiCl4,同时考虑尽可能拟制或降低体系中CaO的条件下,反应气体中Cl2/O2摩尔比值最小为4.00,碳化渣中TiC摩尔质量百分数减小到初始值的28.50%时即可将低温氯化炉内物料转入下一阶段的氯化炉内。
以表1碳化渣中TiC质量百分数13.80%为例计算,氯化反应前后组分含量变化情况如表3所示,可知在第一阶段氯化反应过程中将TiC质量分数从13.80%降低至4.02%时即可转移炉料进行第二阶段氯化反应,此时每100 g碳化渣中的TiC氯化反应消耗的Cl2量为0.39 mol(1.68×0.23 mol)。
表 3 碳化渣加氧氯化不同阶段组分含量变化(初始渣质量以100 g计)Table 3. Changes in component of carbonized slag during different stages of oxychlorination. (The initial slag mass is calculated as 100 g)氯化反
应阶段Cl2/O2
摩尔比混合气体
中Cl2含量/
molTiC初
始量/gTiC初始
量/molTiC残
余量/gTiC残余
量/molCaO初
始量/gCaO初始
量/molCaO残
余量/gCaO残余
量/mol其他组分
含量/gTiC质量
分数/%TiC总氯
化率%CaO总氯
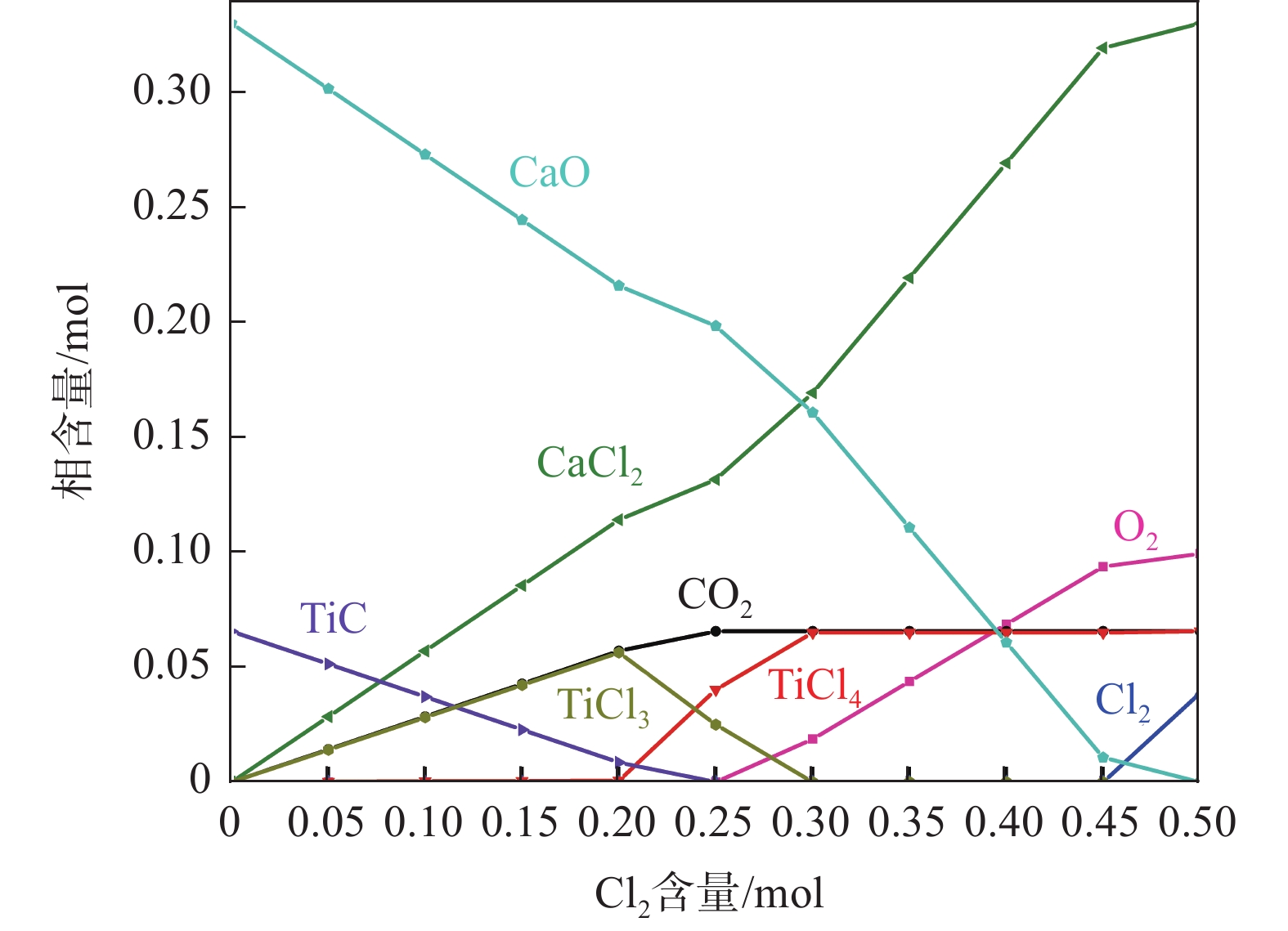
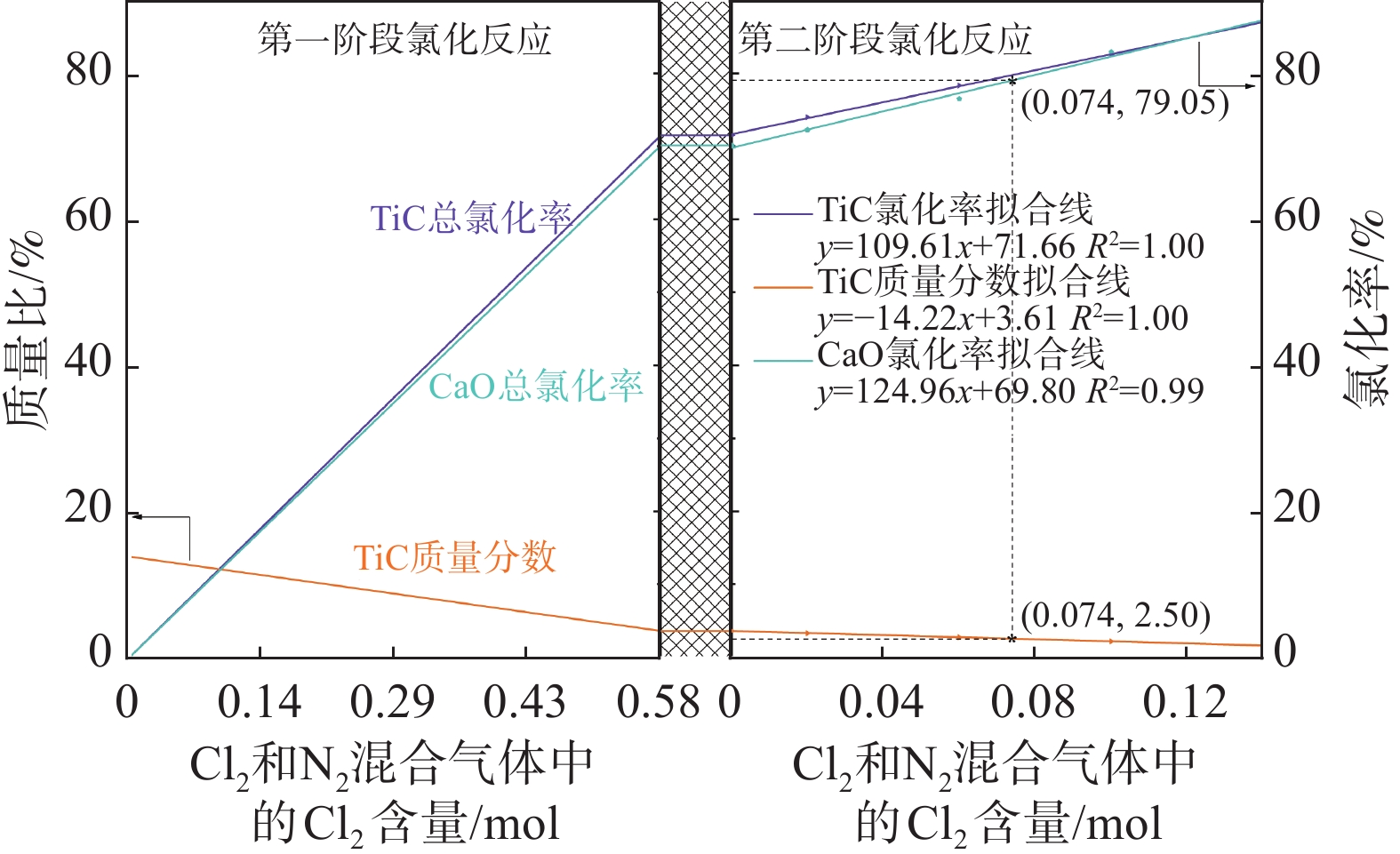
化率/%第一阶段 4.00 13.80 0.23 3.93 0.07 26.57 0.47 18.76 0.33 59.63 4.02 71.50 29.39 第二阶段 0.00 3.93 0.07 3.93 0.07 18.76 0.33 18.76 0.33 59.63 4.02 71.50 29.39 0.02 3.59 0.06 18.11 0.32 3.67 73.99 31.84 0.06 2.99 0.05 16.81 0.30 3.05 78.33 36.73 0.10 2.40 0.04 15.35 0.27 2.41 82.61 42.23 0.14 1.80 0.03 14.21 0.25 1.80 86.96 46.52 在第二阶段氯化反应过程中,由于原料是经过第一阶段氯化后的渣,其中TiC质量分数较低,氯化反应发生后产生的新生碳含量也较少,使得CaO和MgO的加碳氯化程度有限。以第一阶段氯化后渣为原料,第二阶段氯化TiC的反应可采用氯气作为反应气体并混合着N2以混合气体形式进入低温氯化炉内。经过第一阶段氯化反应过后,进行第二阶段氯化反应时TiC和CaO的初始含量如表3所示,根据其初始含量,利用Equilib模块计算得到0.07TiC-0.33CaO-nCl2体系平衡产物含量随Cl2含量变化规律,如图7所示。由图7提取得到不同Cl2含量下TiC的质量分数以及TiC和CaO的氯化率,见表3。随着N2和Cl2混合气体中Cl2比例逐渐增加,渣中TiC被进一步氯化使其质量百分数逐渐降低,而由于CaO也会发生部分氯化反应导致渣中CaO的氯化率也进一步增加。当通入的Cl2总含量与残渣中TiC总含量的摩尔比值大于1.43(0.10/0.07)时,第二阶段氯化反应过后渣中TiC质量百分数可降低至2.41%以下。
为了更加清晰地显示在碳化渣低温氯化反应中是否引入氧气对总Cl2消耗量及CaO氯化的影响,同样以两段氯化反应的方式并以前文所得到的第一阶段中TiC的氯化限度为标准,根据图3计算了碳化渣第一阶段氯化反应未引入氧气(即直接氯化)时一、二阶段氯化反应前后渣中组分及含量变化,结果如表4所示。由图3中TiC含量随Cl2含量变化规律计算出碳化渣直接氯化第一阶段中当TiC含量减小至初始含量的28.50%时,Cl2/TiC摩尔比值为2.50。因此,对于表1中所示的碳化渣实际组分含量推算得到第一阶段氯化反应消耗的Cl2含量为0.58 mol(2.50×0.23 mol)。在碳化渣直接氯化的第二阶段反应中,TiC和CaO的初始含量如表4所示,根据其初始含量,利用Equilib模块计算得到0.07TiC-0.14CaO-nCl2体系平衡产物含量随Cl2含量变化规律,如图8所示。由图8提取得到混合气体中不同Cl2含量下TiC的质量分数以及TiC和CaO的氯化率,见表4。当碳化渣中TiC的氯化率相同时,与表3中碳化渣加氧氯化反应CaO的氯化率相比,直接氯化反应时CaO的氯化率较高。
表 4 碳化渣直接氯化不同阶段组分含量变化(初始渣质量以100 g计)Table 4. Changes in composition and content of slag before and after the first stage chlorination reaction without introducing oxygen. (The initial slag mass is calculated as 100 g)氯化反
应阶段混合气体中
Cl2含量/molTiC初
始量/gTiC初始
量/molTiC残
余量/gTiC残余
量/molCaO初
始量/gCaO初始
量/molCaO残
余量/gCaO残余
量/mol其他组分
含量/gTiC质量
分数/%TiC总氯
化率%CaO总
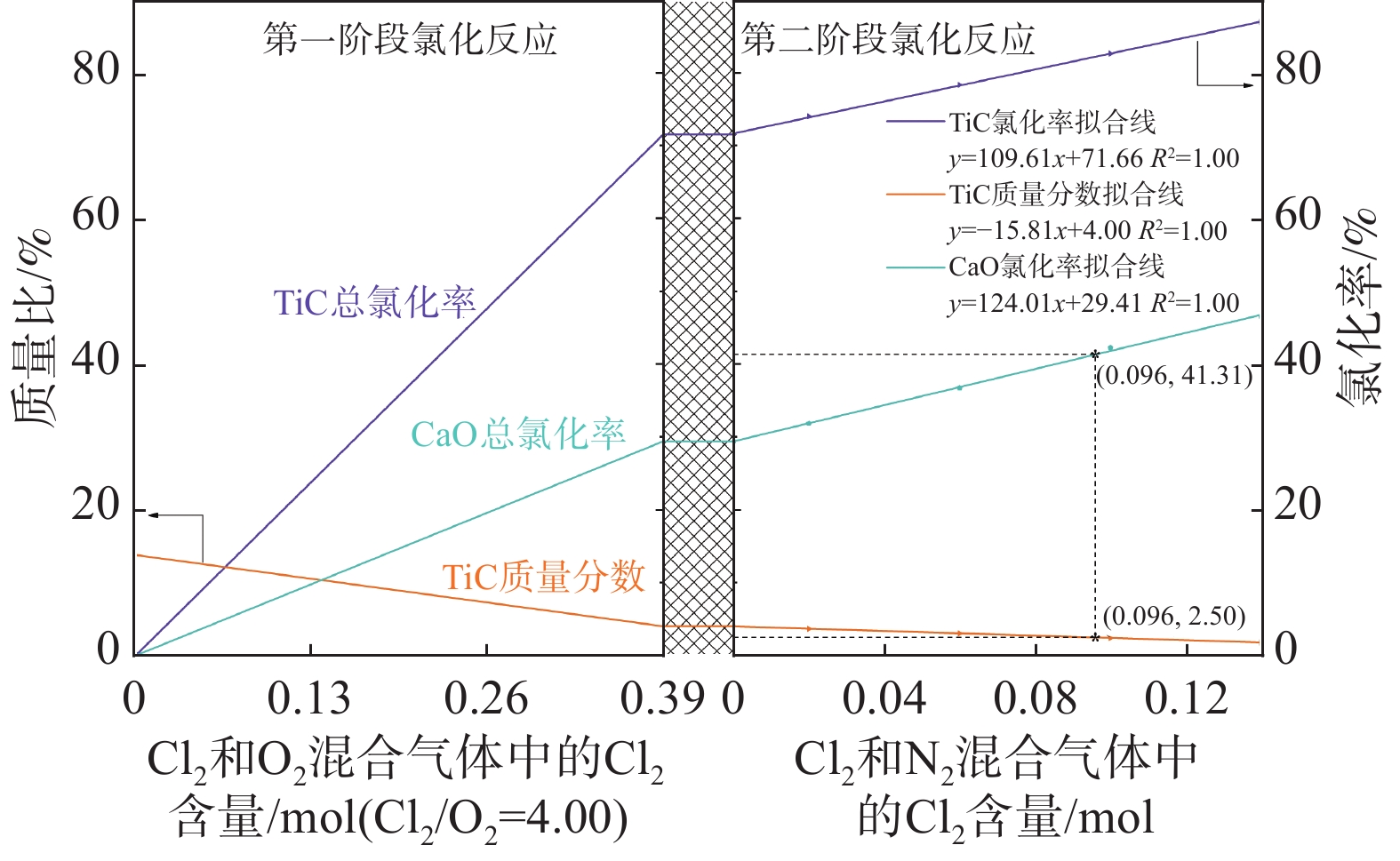
氯化率/%第一阶段 13.80 0.23 3.93 0.07 26.57 0.47 7.94 0.14 59.63 3.63 71.50 70.11 第二阶段 0.00 3.93 0.07 3.93 0.07 7.94 0.14 7.94 0.14 59.63 3.63 71.50 70.11 0.02 3.59 0.06 7.37 0.13 3.31 73.99 72.26 0.06 2.99 0.05 6.24 0.11 2.75 78.33 76.51 0.10 2.40 0.04 4.54 0.08 2.18 82.61 82.91 0.14 1.80 0.03 3.40 0.06 1.63 86.96 87.20 图9和图10分别是表1中TiC和CaO质量分数条件下碳化渣加氧氯化和直接氯化时不同反应阶段TiC质量分数以及TiC和CaO的氯化率随氯化气体中Cl2含量变化的规律。由图9和图10对比可知,当第一阶段氯化反应中碳化渣的氯化率相同时(71.50%),加氧氯化所需的Cl2量(0.39 mol)小于直接氯化所需的Cl2量(0.58 mol),且渣中CaO的氯化率也减小40.72%。当氯化反应完成且渣中TiC质量分数降低至2.50%时,对直接氯化反应而言,第二阶段氯化反应所需的Cl2量为0.074 mol,两个氯化阶段Cl2的总消耗量为0.654 mol;而对加氧氯化反应而言,第二阶段氯化反应所需的Cl2量为0.096 mol,两个氯化阶段Cl2的总消耗量为0.486 mol。当渣中TiC质量分数均降低至2.50%时,加氧氯化比直接氯化Cl2总消耗量减小25.69%((0.654-0.486)/0.654),而渣中CaO总氯化率减小37.74%。
综上可知,在碳化渣低温氯化反应过程中引入适当的氧气不仅可降低反应时氯气消耗量,还可降低渣中CaO的氯化率,与直接氯化相比,对抑制渣中CaO氯化从而达到减缓低温氯化炉内物料粘结问题具有显著优势。
3. 结论
利用Factsage8.1热力学软件分析了引入氧气对碳化钛渣中TiC、CaO和MgO氯化反应的影响,以及平衡物相及含量随氯氧比例的变化规律,通过分析热力学计算结果可得:
1)低温氯化炉内引入氧气可降低碳化渣中TiC氯化反应标准吉布斯自由能,提高TiC氯化反应趋势使其氯化顺序位于CaO和MgO之前,达到选择性氯化提钛目的。
2)在TiC-2.04CaO-0.87MgO-nCl2体系中,当Cl2/TiC摩尔比低于3.50时,MgO不发生氯化反应。在同一Cl2/TiC摩尔比条件下,随着O2含量逐渐升高(即Cl2/O2摩尔比值逐渐减小),TiC氯化率逐渐升高;虽然此时CaO的氯化率也升高,但相较于未引入氧气,CaO的氯化率减少了24.02%~55.39%。
3)在碳化渣加氧分段氯化反应中,第一段氯化反应过程中最佳氯氧气体摩尔比为4.00∶1.00,碳化渣中TiC质量分数从13.80%氯化减少至4.02%时即可结束第一阶段氯化反应;第二阶段氯化反应中无需引入氧气,采用N2+Cl2的混合气体,其中总Cl2含量与总TiC含量摩尔比大于1.43时,可使氯化尾渣中TiC的质量分数降低至2.50%以下。
4)碳化渣加氧氯化反应与直接氯化反应相比,达到相同目标条件(TiC氯化率均为71.50%)时第一阶段氯化反应中CaO氯化率减小40.72%;经过两段氯化反应渣中TiC质量分数降低至2.50%时,加氧氯化比直接氯化Cl2总消耗量减少25.69%,渣中CaO总氯化率减小37.74%。
致谢
论文作者特此感谢攀钢集团有限公司、钒钛资源综合利用产业技术创新战略联盟项目(FTLM2021)“低温氯化流化床多相反应行为及调控研究”的支持。
-
表 1 碳化渣的主要成分及含量
Table 1. Main components and content of carbonized slag
成分 质量分数/% 摩尔数/mol* 摩尔比* TiC 13.80 0.23 1.00 CaO 26.57 0.47 2.04 MgO 8.09 0.20 0.87 SiO2 25.25 0.42 1.83 Al2O3 14.23 0.14 0.61 MnO 0.55 0.01 0.04 Fe 1.00 0.02 0.09 V2O5 0.24 0.01 0.04 TiO2 3.60 0.05 0.22 FeO 2.00 0.03 0.13 C 4.00 0.33 1.43 其它 0.67 *:以100 g碳化渣计的摩尔数和以TiC=1.00 mol为基折算的摩尔比。 表 2 TiC-2.04CaO-0.87MgO-nCl2-mO2反应体系中各物相氯化率随O2含量变化规律
Table 2. The variation of chlorination rates of various phases with O2 contents in the TiC-2.04CaO-0.87MgO-nCl2-mO2 reaction system
Cl2含量n TiC氯化率/% CaO氯化率/% 最优O2/TiC 摩尔比 Cl2/O2摩尔比 未引入氧气 引入氧气 氯化率变化情况 未引入氧气 引入氧气 氯化率变化情况 1.80 51.35 100.00 +48.65 50.33 0.00 −50.33 1.10 1.64 1.90 54.20 100.00 +45.80 53.12 0.00 −53.12 1.10 1.73 2.00 57.06 100.00 +42.94 55.88 0.49 −55.39 1.00 2.00 2.10 59.91 100.00 +40.09 58.82 5.39 −53.43 1.00 2.10 2.20 62.76 100.00 +37.24 61.27 10.29 −50.98 0.90 2.44 2.30 65.62 100.00 +34.38 64.22 15.20 −49.02 0.90 2.56 2.40 68.47 100.00 +31.53 67.16 20.10 −47.06 0.80 3.00 2.50 71.32 100.00 +28.68 70.10 25.00 −45.10 0.80 3.13 2.70 77.03 100.00 +22.97 75.49 34.80 −40.69 0.70 3.86 2.90 82.74 100.00 +17.26 80.88 44.61 −36.27 0.60 4.83 3.10 88.44 100.00 +11.56 86.76 54.41 −32.35 0.50 6.20 3.30 94.15 100.00 +5.85 92.16 64.22 −27.94 0.40 8.25 3.50 100.00 100.00 +0.00 98.04 74.02 −24.02 0.30 11.67 表 3 碳化渣加氧氯化不同阶段组分含量变化(初始渣质量以100 g计)
Table 3. Changes in component of carbonized slag during different stages of oxychlorination. (The initial slag mass is calculated as 100 g)
氯化反
应阶段Cl2/O2
摩尔比混合气体
中Cl2含量/
molTiC初
始量/gTiC初始
量/molTiC残
余量/gTiC残余
量/molCaO初
始量/gCaO初始
量/molCaO残
余量/gCaO残余
量/mol其他组分
含量/gTiC质量
分数/%TiC总氯
化率%CaO总氯
化率/%第一阶段 4.00 13.80 0.23 3.93 0.07 26.57 0.47 18.76 0.33 59.63 4.02 71.50 29.39 第二阶段 0.00 3.93 0.07 3.93 0.07 18.76 0.33 18.76 0.33 59.63 4.02 71.50 29.39 0.02 3.59 0.06 18.11 0.32 3.67 73.99 31.84 0.06 2.99 0.05 16.81 0.30 3.05 78.33 36.73 0.10 2.40 0.04 15.35 0.27 2.41 82.61 42.23 0.14 1.80 0.03 14.21 0.25 1.80 86.96 46.52 表 4 碳化渣直接氯化不同阶段组分含量变化(初始渣质量以100 g计)
Table 4. Changes in composition and content of slag before and after the first stage chlorination reaction without introducing oxygen. (The initial slag mass is calculated as 100 g)
氯化反
应阶段混合气体中
Cl2含量/molTiC初
始量/gTiC初始
量/molTiC残
余量/gTiC残余
量/molCaO初
始量/gCaO初始
量/molCaO残
余量/gCaO残余
量/mol其他组分
含量/gTiC质量
分数/%TiC总氯
化率%CaO总
氯化率/%第一阶段 13.80 0.23 3.93 0.07 26.57 0.47 7.94 0.14 59.63 3.63 71.50 70.11 第二阶段 0.00 3.93 0.07 3.93 0.07 7.94 0.14 7.94 0.14 59.63 3.63 71.50 70.11 0.02 3.59 0.06 7.37 0.13 3.31 73.99 72.26 0.06 2.99 0.05 6.24 0.11 2.75 78.33 76.51 0.10 2.40 0.04 4.54 0.08 2.18 82.61 82.91 0.14 1.80 0.03 3.40 0.06 1.63 86.96 87.20 -
[1] Ahmadi E, Rezan S A, Baharun N, et al. Chlorination kinetics of titanium nitride for production of titanium tetrachloride from nitrided ilmenite[J]. Metallurgical and Materials Transactions B, 2017,48(5):2354−2366. doi: 10.1007/s11663-017-1011-z [2] Yang F, Wen L Y, Yue D, et al. Study on reaction behaviors and mechanisms of rutile TiO2 with different carbon addition in fluidized chlorination[J]. Journal of Materials Research and Technology, 2022,18:1205−1217. doi: 10.1016/j.jmrt.2022.02.131 [3] Zhu F X, Qiu K H, Sun Z H. Preparation of titanium from TiCl4 in a molten fluoride-chloride salt[J]. Electrochemistry, 2017,85(11):715−720. doi: 10.5796/electrochemistry.85.715 [4] Shi J J, Qiu Y C, Yu B, et al. Titanium extraction from titania-bearing blast furnace slag: A review[J]. American Journal of Respiratory and Critical Care Medicine, 2022,74(2):654−667. [5] Qin J, Wang Y, You Z X, et al. Carbonization and nitridation of vanadium–bearing titanomagnetite during carbothermal reduction with coal[J]. Journal of Materials Research and Technology, 2020,9(3):4272−4282. doi: 10.1016/j.jmrt.2020.02.053 [6] Peng Yi. Thermodynamic analysis on the selective chlorination of carbonized Pangang BF slag at low temperature[J]. Titanium Industry Progress, 2005,6:45−49. (彭毅. 碳化攀钢高炉渣低温选择氯化的热力学分析[J]. 钛工业进展, 2005,6:45−49. doi: 10.3969/j.issn.1009-9964.2005.01.013Peng Yi. Thermodynamic analysis on the selective chlorination of carbonized Pangang BF slag at low temperature [J]. Titanium Industry Progress, 2005, 6: 45-49. doi: 10.3969/j.issn.1009-9964.2005.01.013 [7] 刘晓华. 改性含钛高炉渣高温碳化低温氯化的研究[D]. 沈阳: 东北大学, 2009.Liu Xiaohua. Study on high-temperature carbonization and low-temperature chlorination on modified titanium bearing blast furnace slag[D]. Shengyang: Northeastern University, 2009. [8] Taki T, Komoto S, Otomura K, et al. Chloride pyrometallurgy of uranium ore (II)[J]. Journal of Nuclear Science and Technology, 1996,33(4):327−332. doi: 10.1080/18811248.1996.9731912 [9] Hiroyuki M, Fumitaka T. Chlorination kinetics of ZnO with Ar-Cl2-O2 gas and the effect of oxychloride formation[J]. Metallurgical and Materials Transactions B, 2006,37(3):413−420. doi: 10.1007/s11663-006-0026-7 [10] Hiroyuki M, Tasuku H, Fumitaka T. Chlorination kinetics of ZnFe2O4 with Ar-Cl2-O2 gas[J]. Materials Transactions, 2006,47(10):2524−2532. doi: 10.2320/matertrans.47.2524 [11] Jungshin K, Toru H O. Removal of iron from titanium ore by selective chlorination using TiCl4 under high oxygen chemical potential[J]. International Journal of Mineral Processing, 2016,149:111−118. doi: 10.1016/j.minpro.2016.02.014 -





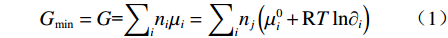
 下载:
下载:













 下载:
下载: