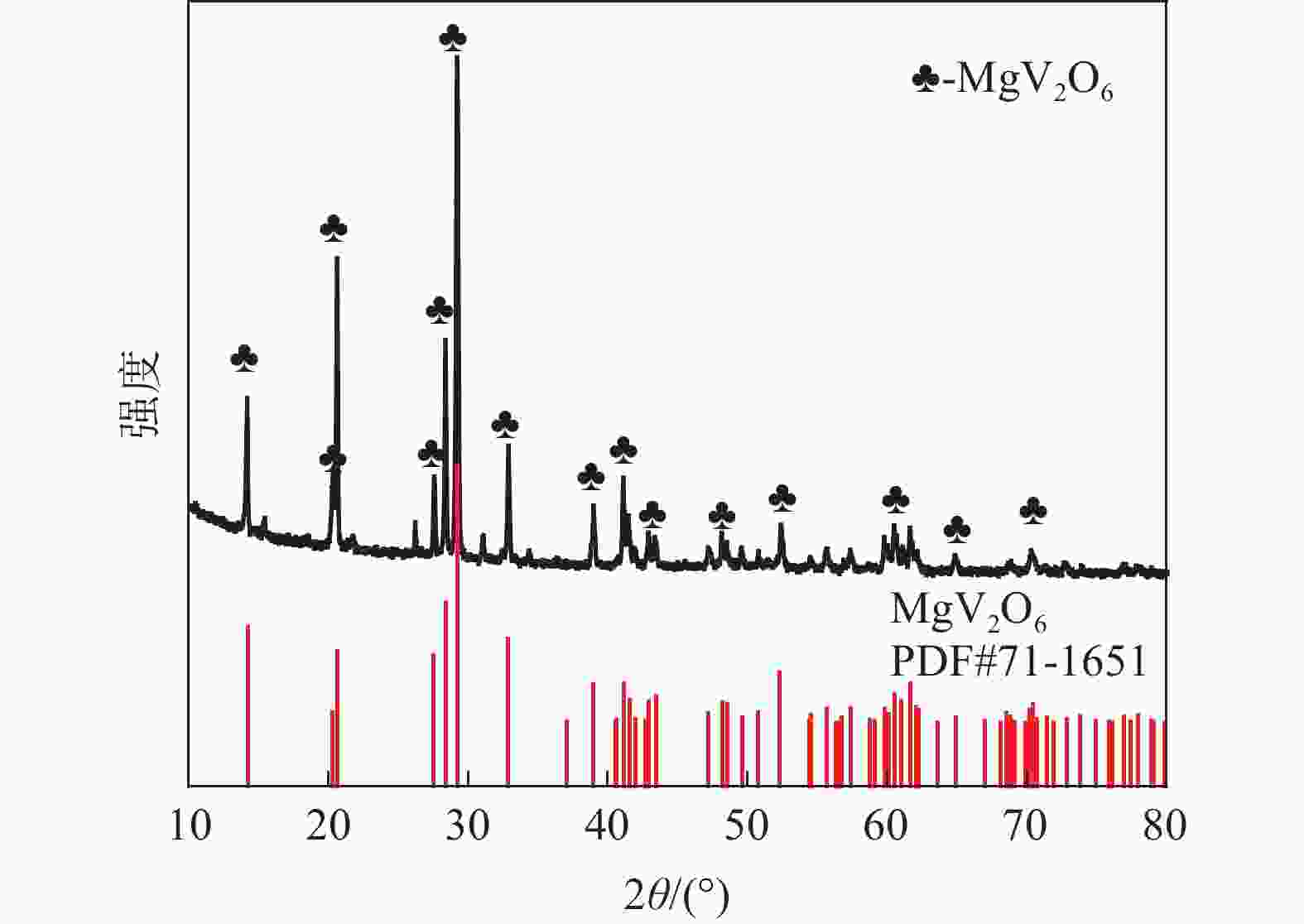
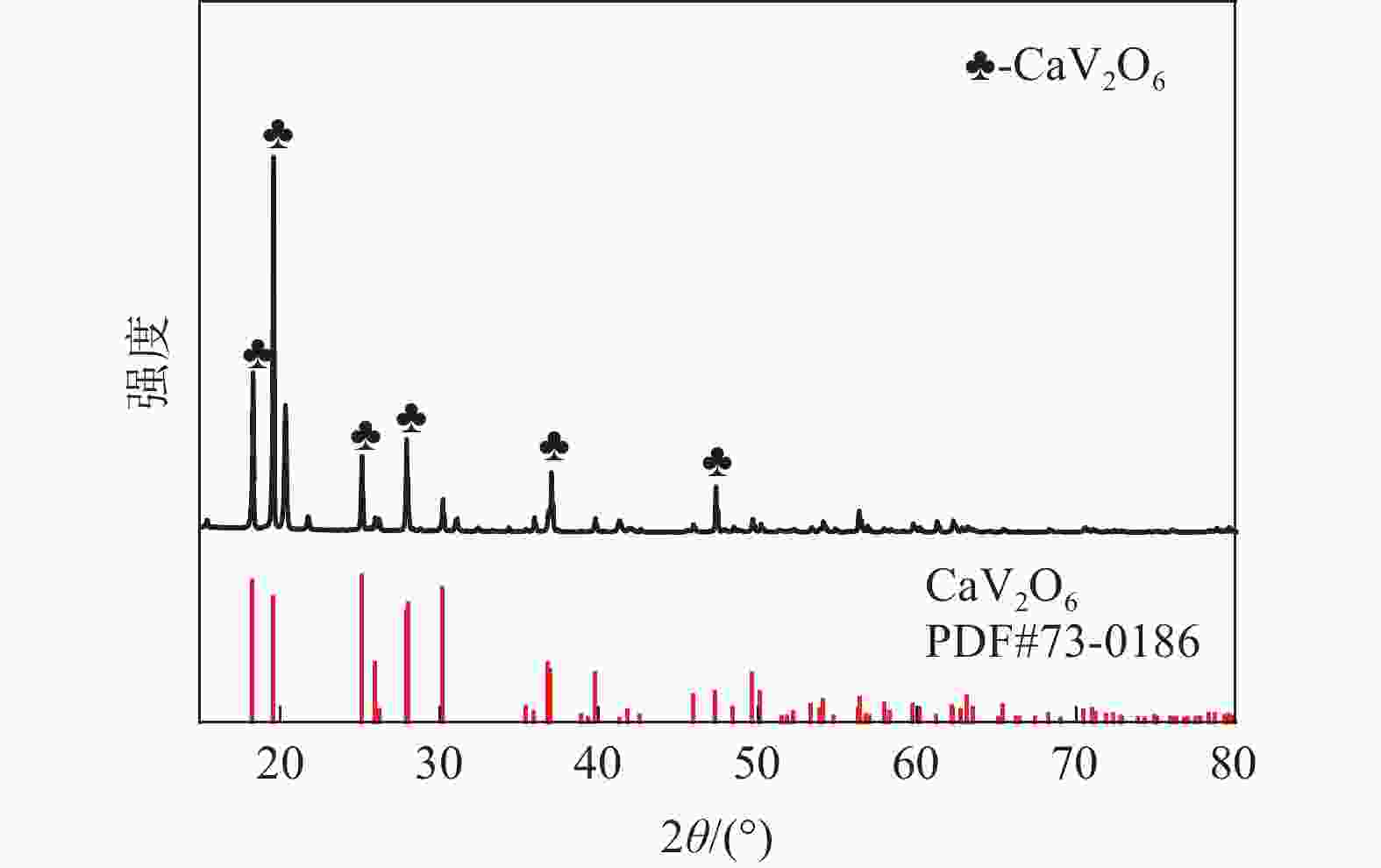
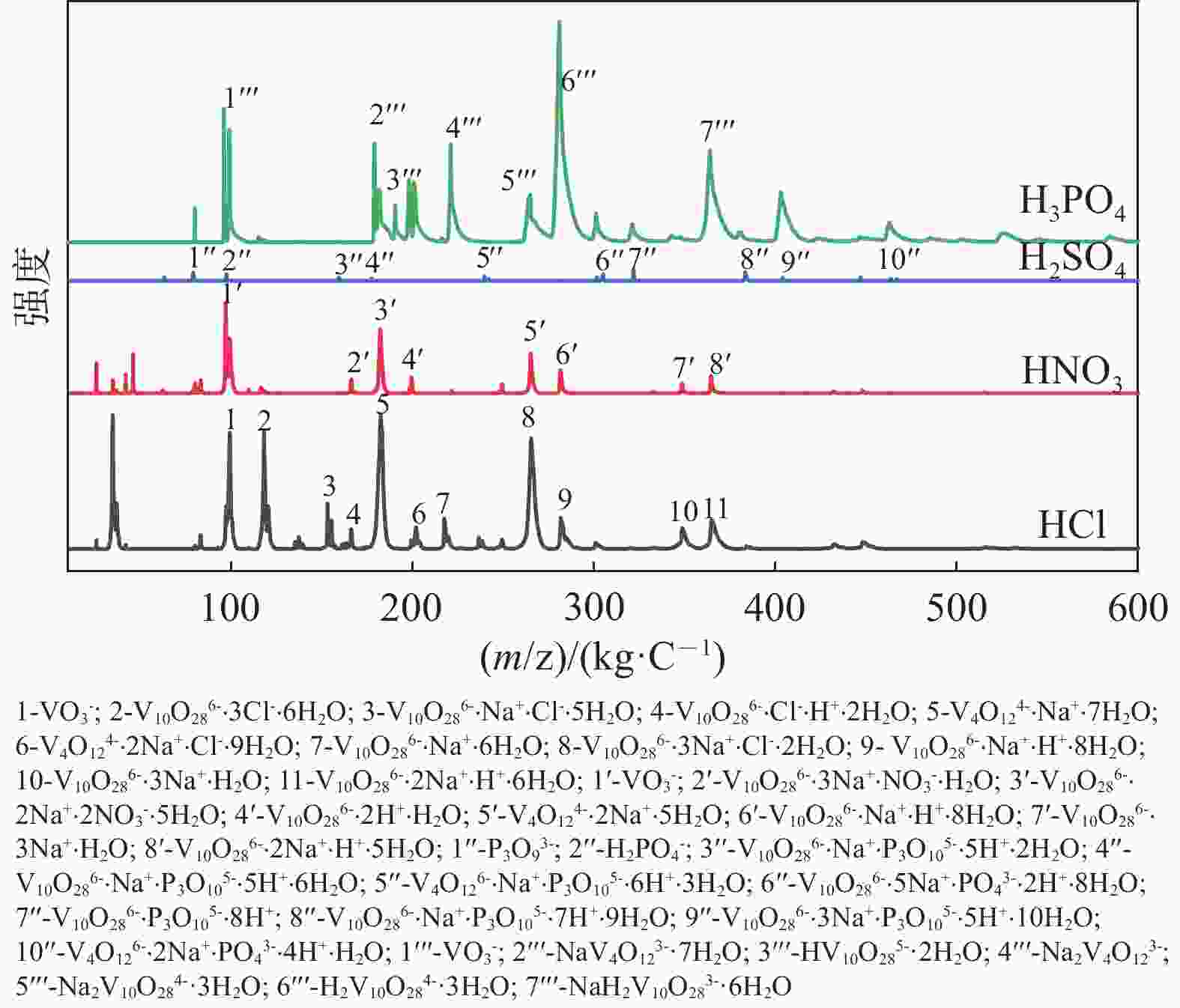
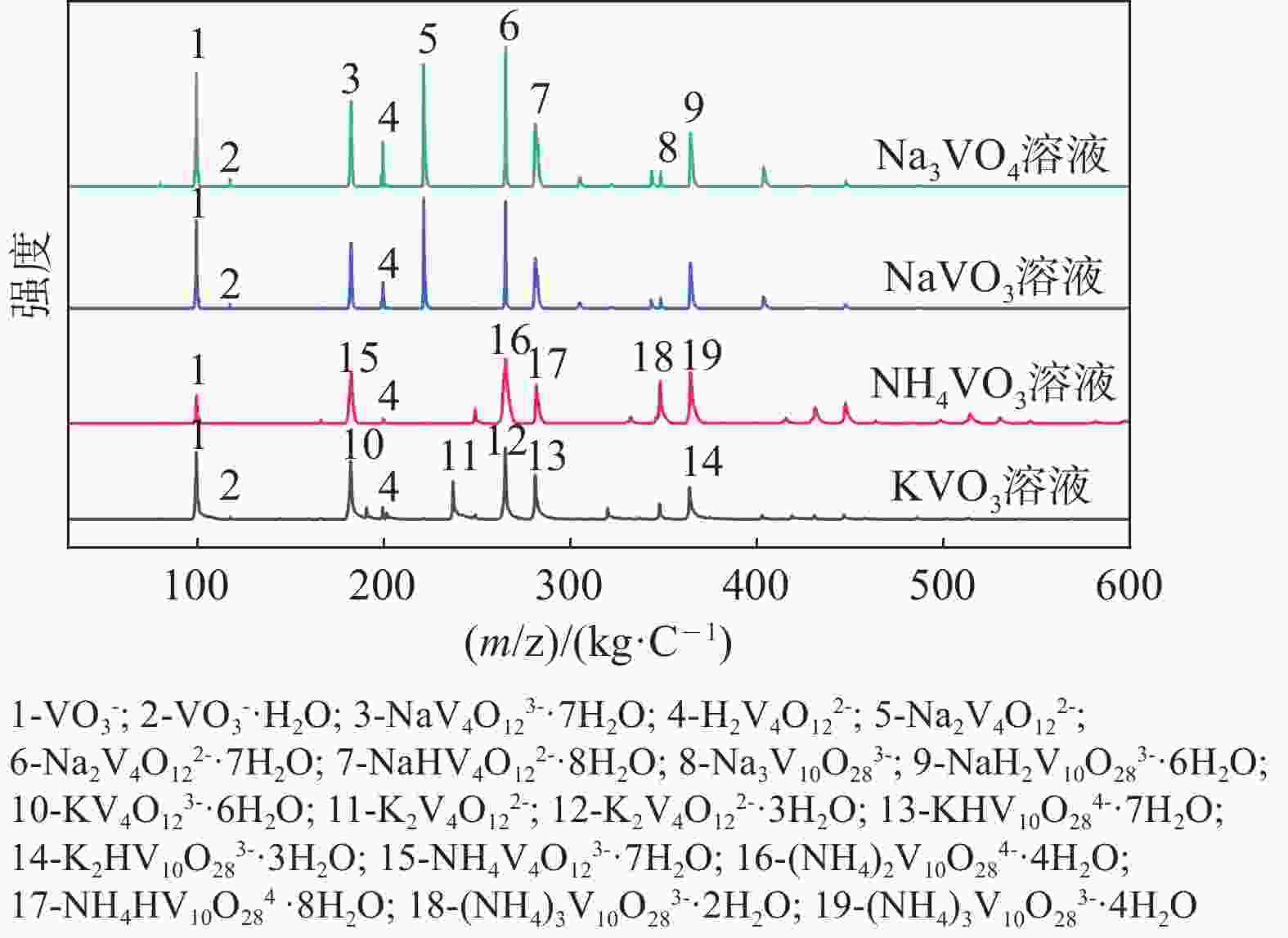
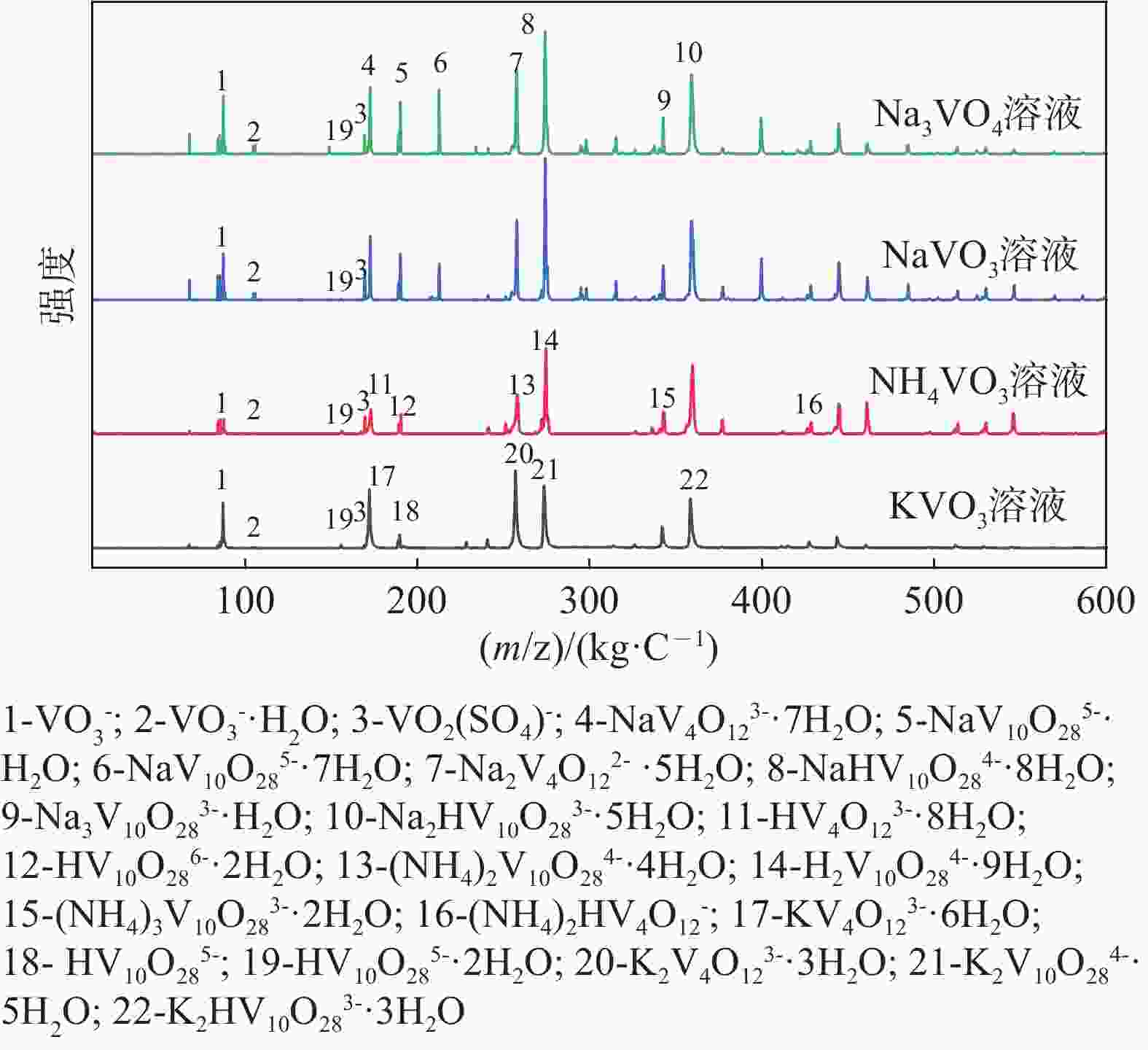
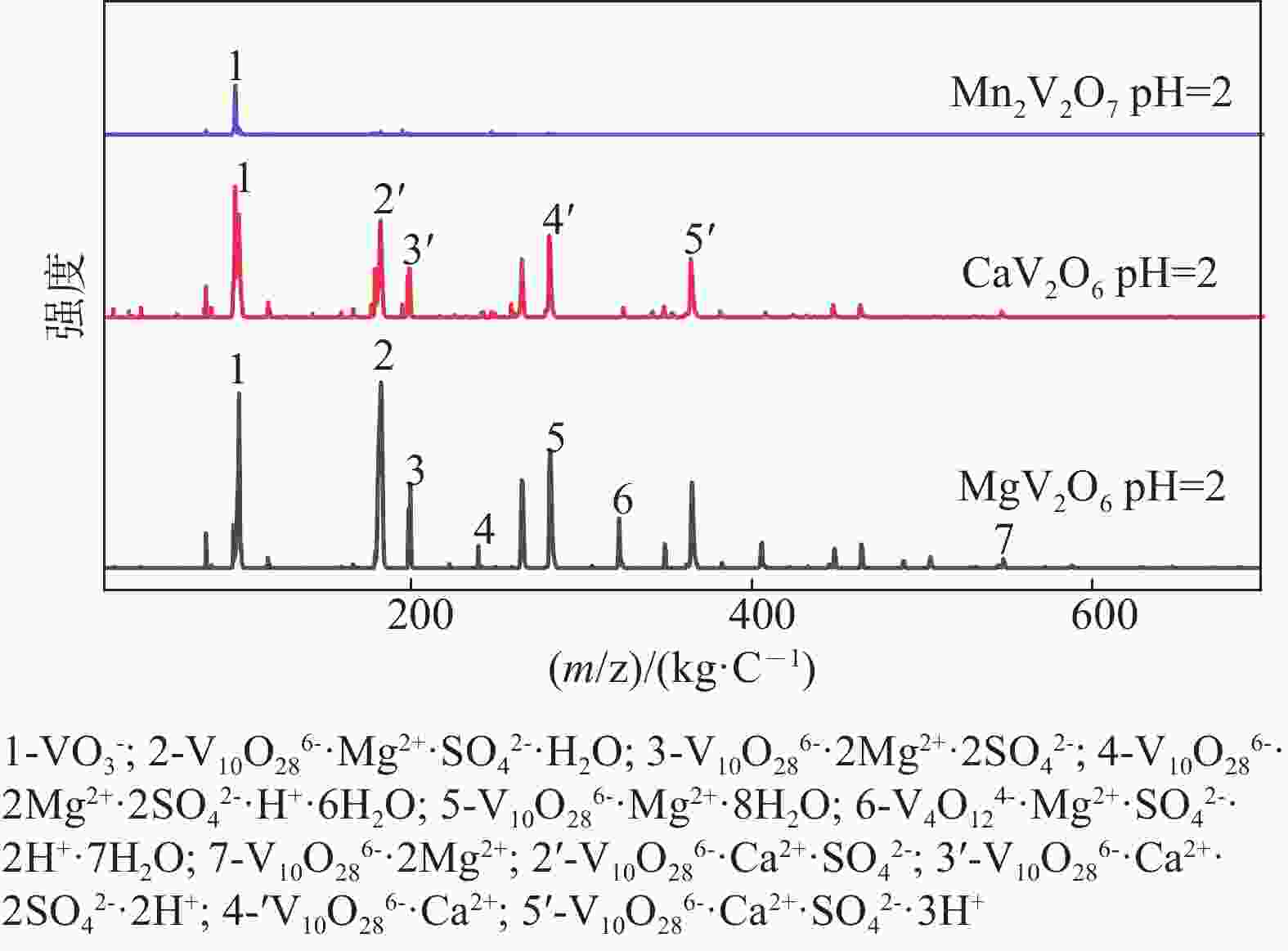
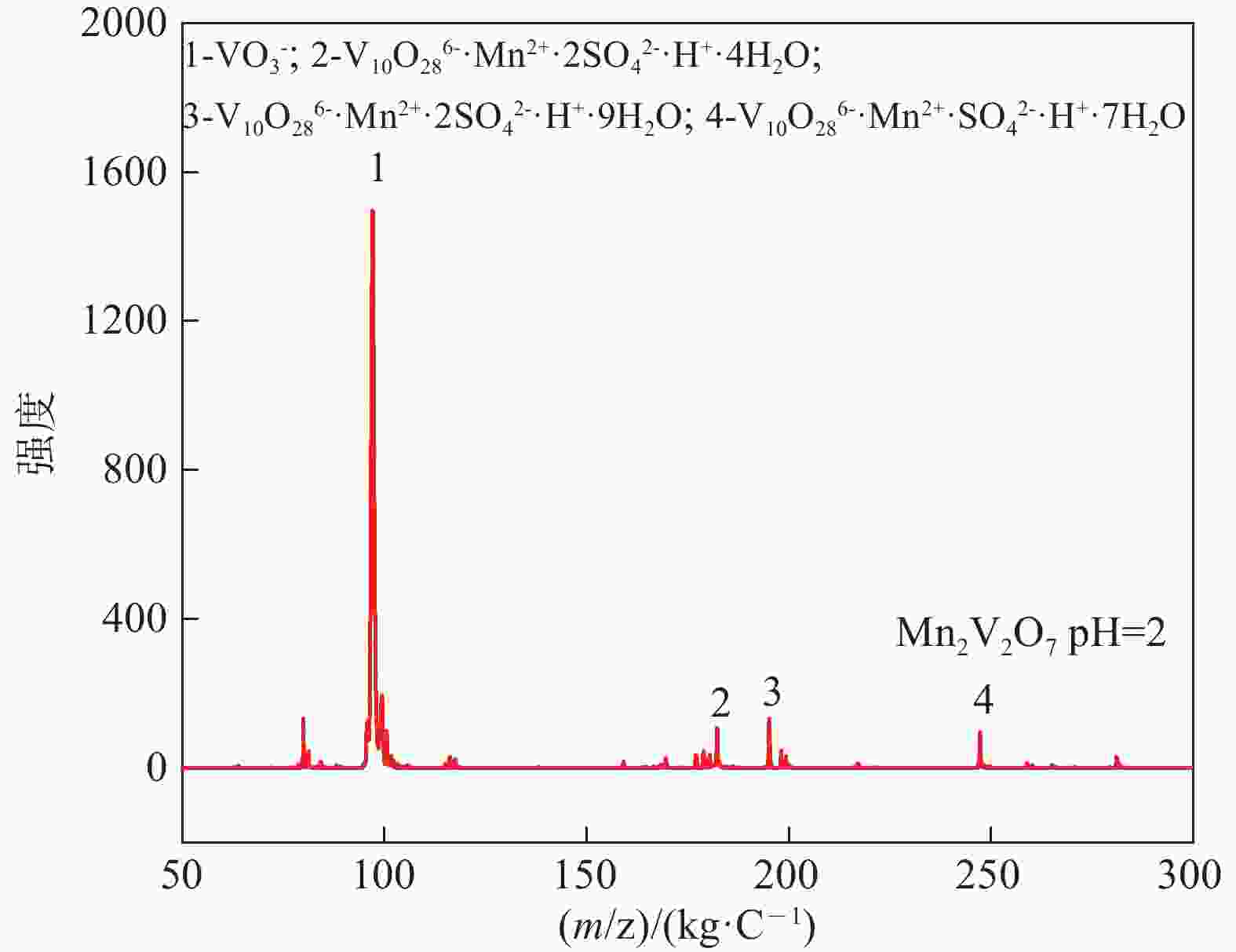
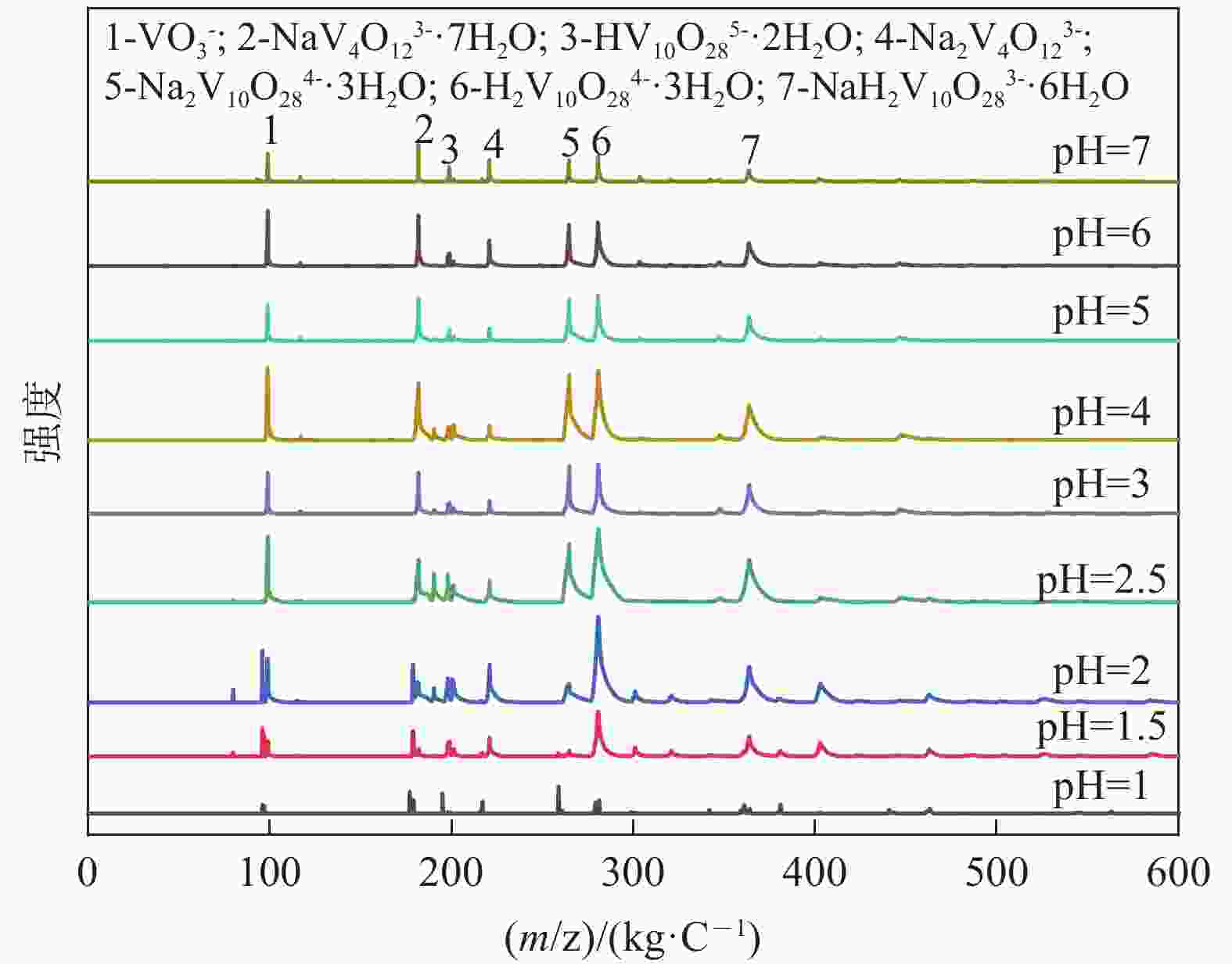
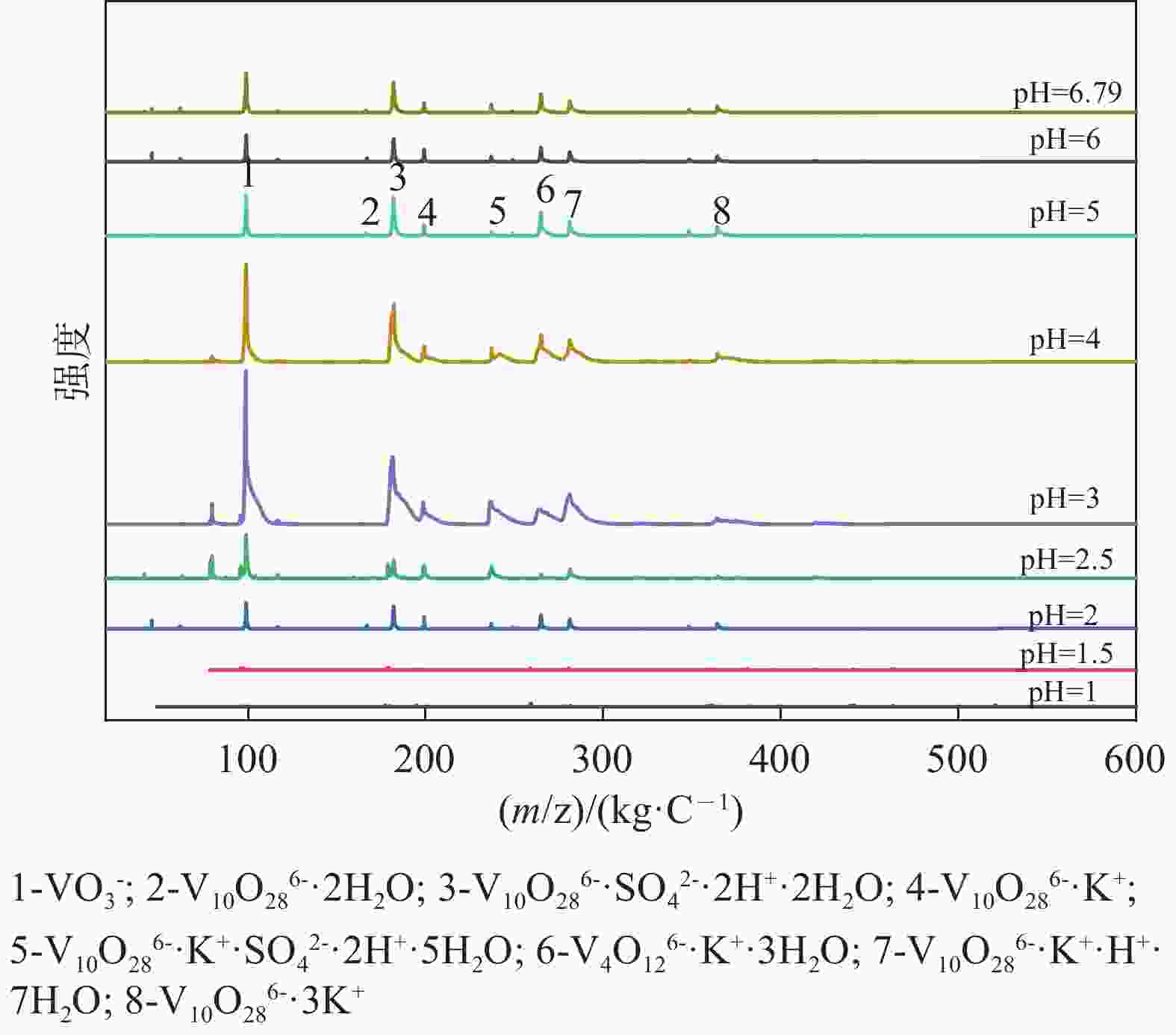
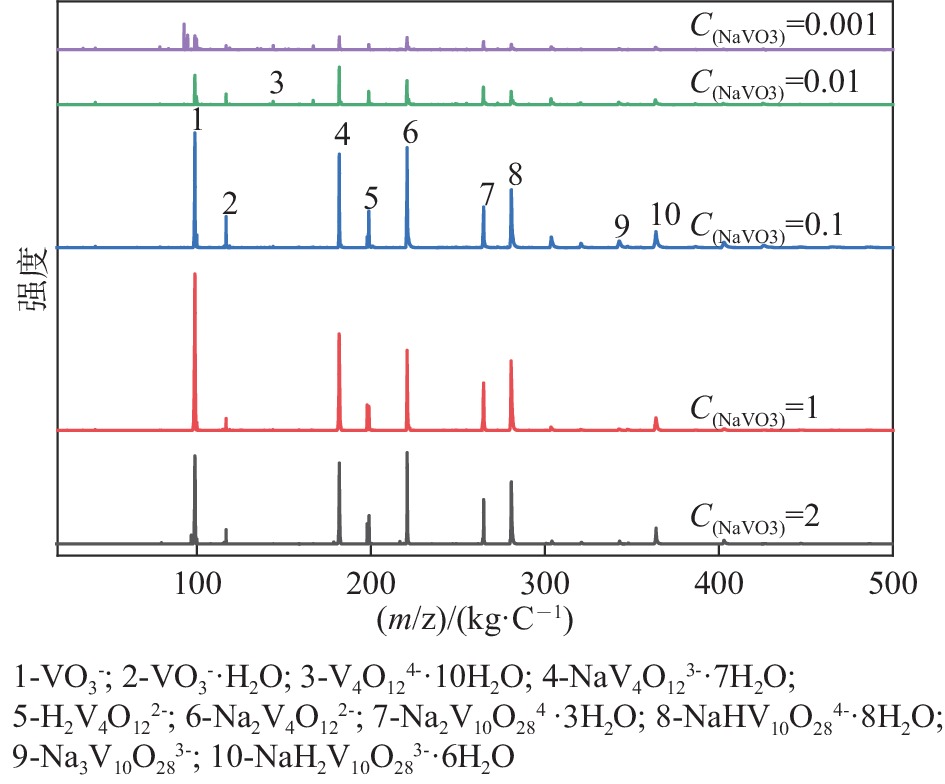
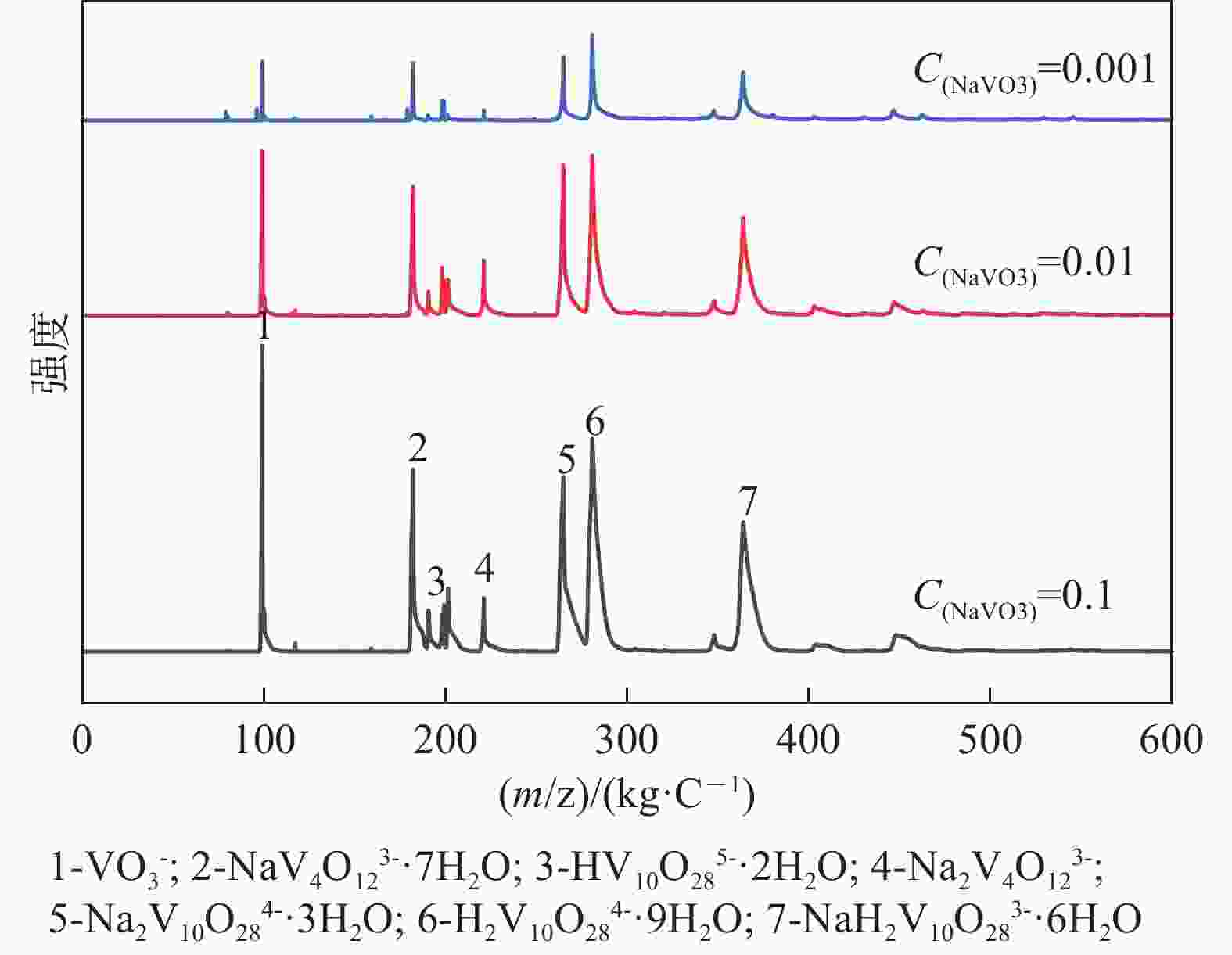
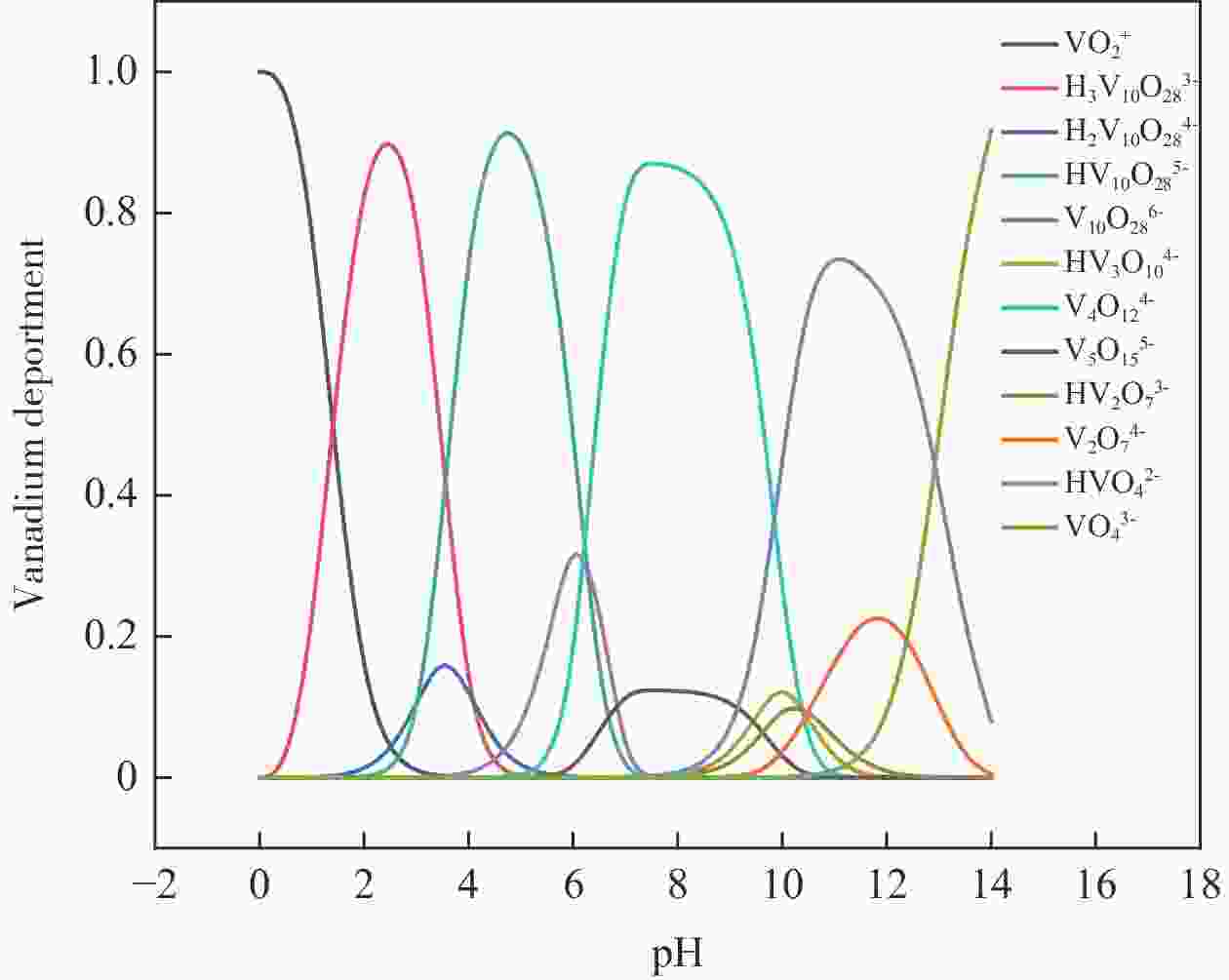
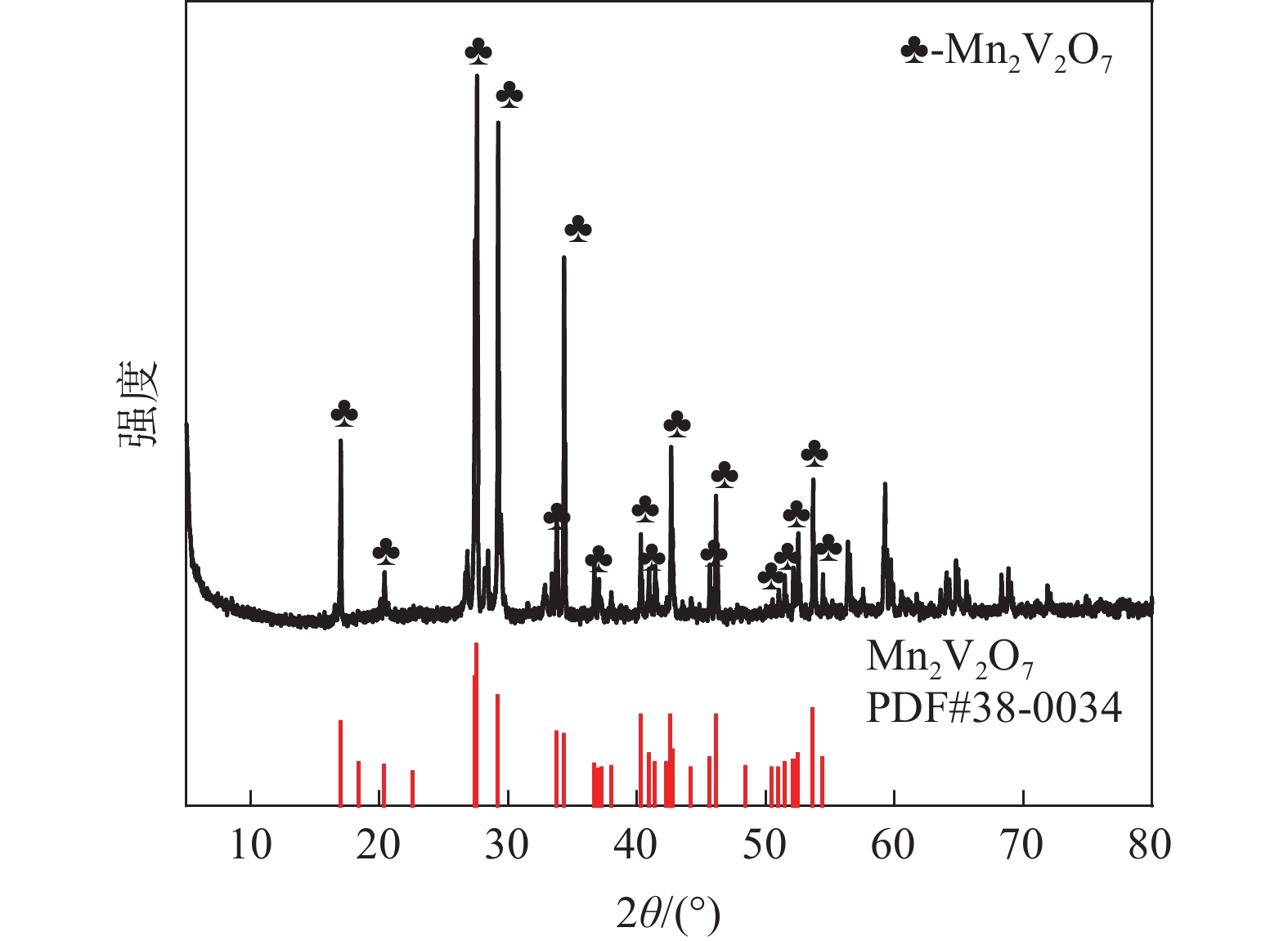
| [1] |
Li H Y, Wang C J, Yuan Y H, et al. Magnesiation roasting-acid leaching: A zero-discharge method for vanadium extraction from vanadium slag[J]. J Clean Prod, 2020,260:121091. doi: 10.1016/j.jclepro.2020.121091
|
| [2] |
Zhu X B, Li W, Zhang C X. Extraction and removal of vanadium by adsorption with resin 201*7 from vanadium waste liquid[J]. Environ Res, 2020,180:108865. doi: 10.1016/j.envres.2019.108865
|
| [3] |
Teng A J, Xue X X. A novel roasting process to extract vanadium and chromium from high chromium vanadium slag using a NaOH−NaNO3 binary system[J]. J Hazard Mater, 2019,379:120805. doi: 10.1016/j.jhazmat.2019.120805
|
| [4] |
Xu Zhengzhen, Liang Jinglong, Li Hui, et al. Research status and prospects of vanadium recovery in vanadium containing wastes[J]. Multipurpose Util Miner Resour, 2020,(3):8−13. (徐正震, 梁精龙, 李慧, 等. 含钒废弃物中钒的回收研究现状及展望[J]. 矿产综合利用, 2020,(3):8−13.Xu Z Z, Liang J L, Li H, et al. Research status and prospects of vanadium recovery in vanadium containing wastes [J]. Multipurpose Util Miner Resour, 2020(3): 8.
|
| [5] |
Wu Enhui, Hou Jing, Li Jun. Experimental study on vanadium extraction from vanadium and chromium slag by oxidizing and calcifying roasting and acid leaching[J]. Rare Metals and Cemented Carbide, 2017,45(6):8−13. (吴恩辉, 侯静, 李军. 钒铬渣氧化钙化焙烧-酸浸提钒实验研究[J]. 稀有金属与硬质合金, 2017,45(6):8−13.Wu Enhui, Hou Jing, Li Jun. Experimental study on vanadium extraction from vanadium and chromium slag by oxidizing and calcifying roasting and acid leaching[J]. Rare Metals and Cemented Carbide, 2017, 45 (6): 8-13.
|
| [6] |
Xiao Xiudi, Zhang Hua, Chai Guanqi, et al. A cost-effective process to prepare VO2 (M) powder and films with superior thermochromic properties[J]. Materials Research Bulletin, 2014,51:6−12. doi: 10.1016/j.materresbull.2013.11.051
|
| [7] |
Johann Nathan Nicholas, Gabriel Da Silva, Sandra Kentish, et al. Use of vanadium (V) oxide as a catalyst for CO2 hydration in potassium carbonate systems[J]. Industrial & Engineering Chemistry Research, 2014,53:3029−3039.
|
| [8] |
Wu Gao, Pan Peng, Fan Helin, et al. Research status and development trend of vanadium extraction from vanadium slag[J]. Jiangxi Metallurgy, 2020,40(4):19−27. (吴诰, 潘鹏, 范鹤林, 等. 钒渣提钒研究现状及发展趋势[J]. 江西冶金, 2020,40(4):19−27.Wu Gao, Pan Peng, Fan Helin, et al. Research status and development trend of vanadium extraction from vanadium slag [J]. Jiangxi Metallurgy, 2020, 40 (4): 19-27.
|
| [9] |
Hong Ying, Guo Shuanghua, Li Yu, et al. Progress in vanadium extraction technology[J]. Guangzhou Chemical Industry, 2021,49(17):23−25. (洪颖, 郭双华, 李雨, 等. 提钒技术研究进展[J]. 广州化工, 2021,49(17):23−25. doi: 10.3969/j.issn.1001-9677.2021.17.008Hong Ying, Guo Shuanghua, Li Yu, et al. Progress in vanadium extraction technology [J]. Guangzhou Chemical Industry, 2021, 49 (17): 23-25. doi: 10.3969/j.issn.1001-9677.2021.17.008
|
| [10] |
Xie Yu, Ye Guohua, Zuo Qi, et al. Study on the new process of vanadium extraction from vanadium-bearing steel slag[J]. Iron Steel Vanadium Titanium, 2019,40(1):69−77. (谢禹, 叶国华, 左琪, 等. 含钒钢渣提钒新工艺研究[J]. 钢铁钒钛, 2019,40(1):69−77.Xie Yu, Ye Guohua, Zuo Qi, et al. Study on the new process of vanadium extraction from vanadium-bearing steel slag [J]. Iron Steel Vanadium Titanium, 2019, 40 (1): 69-77
|
| [11] |
Chen Dongli, Tang Cheng. Research on the influence of vanadium precipitation conditions on vanadium precipitation rate[J]. Equipment Manufacturing Technology, 2016,(1):260−262. (陈冬丽, 唐铖. 沉钒条件对沉钒率的影响研究[J]. 装备制造技术, 2016,(1):260−262. doi: 10.3969/j.issn.1672-545X.2016.01.087Chen Dongli, Tang Cheng. Research on the influence of vanadium precipitation conditions on vanadium precipitation rate [J]. Equipment Manufacturing Technology, 2016 (1): 260-262. doi: 10.3969/j.issn.1672-545X.2016.01.087
|
| [12] |
Zhang Juhua, Yan Zhefeng, Zhang Li. Influencing factors and kinetics of vanadium precipitation from calcified vanadium extraction solution[J]. Journal of Process Engineering, 2018,18(1):111−117. (张菊花, 严哲锋, 张力. 钙化提钒溶液沉钒的影响因素及沉钒动力学[J]. 过程工程学报, 2018,18(1):111−117.Zhang Juhua, Yan Zhefeng, Zhang Li. Influencing factors and kinetics of vanadium precipitation from calcified vanadium extraction solution [J]. Journal of Process Engineering, 2018, 18 (1): 111-117.
|
| [13] |
Zhang J H, Zhang W, Zhang L, et al. Mechanism of vanadium slag roasting with calcium oxide[J]. International Journal of Mineral Processing, 2015,138:20−29. doi: 10.1016/j.minpro.2015.03.007
|
| [14] |
Zhang J H, Zhang W, Xue Z L. Oxidation kinetics of vanadium slag roasting in the presence of calcium oxide[J]. Mineral Processing and Extractive Metallurgy Review, 2017,38(5):265−273. doi: 10.1080/08827508.2017.1289197
|
| [15] |
Liu H B, Du H, Wang D W, et al. Kinetics analysis of decomposition of vanadium slag by KOH sub-molten salt method[J]. Transactions of Nonferrous Metals Society of China, 2013,23(5):1489−1500. doi: 10.1016/S1003-6326(13)62621-7
|
| [16] |
Zhang G Q, Zhang T A, Li G Z, et al. Extraction of vanadium from LD converter slag by pressure leaching process with titanium white waste acid[J]. Rare Metal Materials Engineering, 2015,44(8):1894−1898. doi: 10.1016/S1875-5372(15)30120-X
|
| [17] |
Liu Z H, Li Y, Chen M L, et al. Enhanced leaching of vanadium slag in acidic solution by eletro-oxidation[J]. Hydrometallurgy, 2016,159:1−5.
|
| [18] |
Yu Tangxia, Wen Jing, Sun Hongyan, et al. Effect of phase structure of vanadium slag with different calcium content on vanadium extraction by calcification[J]. Iron Steel Vanadium Titanium, 2021,42(5):18−23. (余唐霞, 温婧, 孙红艳, 等. 不同钙含量钒渣的物相结构对钙化提钒的影响[J]. 钢铁钒钛, 2021,42(5):18−23.Yu Tangxia, Wen Jing, Sun Hongyan, et al. Effect of phase structure of vanadium slag with different calcium content on vanadium extraction by calcification [J] . Iron Steel Vanadium Titanium, 2021, 42 (5): 18-23.
|
| [19] |
Tao Changyuan, Yu Qiang, Liu Zuohua, et al. Directional conversion and removal of complex phosphorus compounds from acidic leaching solution containing vanadium[J]. Journal of Water Process Engineering, 2020,37:101−131.
|
| [20] |
Liu Hong, Zhang Yimin, Huang Jing. N235 supported liquid membrane separation of vanadium extraction from shale and mass transfer mechanism[J]. Chinese Journal of Nonferrous Metals, 2020,30(9):2216−2223. (刘红, 张一敏, 黄晶. N235支撑液膜分离页岩提钒酸浸液及传质机理[J]. 中国有色金属学报, 2020,30(9):2216−2223.Liu Hong, Zhang Yimin, Huang Jing. N235 supported liquid membrane separation of vanadium extraction from shale and mass transfer mechanism [J]. Chinese Journal of Nonferrous Metals, 2020, 30 (9): 2216-2223.
|
| [21] |
Selling A, Andersson I, Pettersson L, et al. Multicomponent polyanions. 47. The aqueous vanadophosphate system[J]. Inorganic Chemistry, 2002,33(14):3141−3150.
|
| [22] |
Zhang Weiguang, Zhang Ting,an, Lü Guozhi, et al. Thermodynamic study on the V(V)-P(V)-H2O system in acidic leaching solution of vanadium-bearing converter slag[J]. Separation & Purification Technology, 2019,218:164−172.
|
| [23] |
Zhang Weiguang, Zhang Ting'an, Sun Ying, et al. Thermodynamic analysis of vanadium extraction from acidic solution of phosphorus-sulfur-vanadium-water system[J]. Rare Metal Materials and Engineering, 2021,50(12):4265−4271. (张伟光, 张廷安, 孙颖, 等. 磷-硫-钒-水体系酸性溶液中钒的提取热力学分析[J]. 稀有金属材料与工程, 2021,50(12):4265−4271.Zhang Weiguang, Zhang Ting'an, Sun Ying, et al. Thermodynamic analysis of vanadium extraction from acidic solution of phosphorus-sulfur-vanadium-water system [J]. Rare Metal Materials and Engineering, 2021, 50 (12): 4265-4271.
|
| [24] |
Zhang J H, Zhang W, Zhang L, et al. A critical review of technology for selective recovery of vanadium from leaching solution in V2O5 production[J]. Solvent Extraction and Ion Exchange, 2014,32(3):221−248. doi: 10.1080/07366299.2013.877753
|
| [25] |
Zhang J H, Zhang W, Xue Z L. An environment-friendly process featuring calcified roasting and precipitation purification to prepare vanadium pentoxide from the converter vanadium slag[J]. Metals, 2018,9(1):103390.
|
| [26] |
肖亮. 钒溶液沉钒工艺研究进展 [C]// 第三届钒产业先进技术研讨与交流会论文集. 西昌: 钒钛资源综合利用产业技术创新战略联盟, 钒钛资源综合利用国家重点实验室, 2015.Xiao Liang. Research progress of vanadium solution vanadium precipitation process [C]//Proceedings of the 3rd Vanadium Industry Advanced Technology Seminar and Exchange Conference. Xichang: Strategic Alliance for Technological Innovation of Vanadium and Titanium Resources Comprehensive Utilization Industry, State Key Laboratory of Vanadium and Titanium Resources Comprehensive Utilization, 2015.
|
| [27] |
杨保祥, 何金勇, 张桂芳. 钒基材料制造[M]. 北京: 冶金工业出版社, 2014: 104.Yang Baoxiang, He Jinyong, Zhang Guifang. Vanadium-based material manufacturing[M]. Beijing: Metallurgical Industry Press, 2014: 104.
|
| [28] |
Murthy G V R, Reddy T S, Rao S B. Extraction and simultaneous spectro photometric determination of vanadium(IV) and vanadium(V) in admixture with 2-hydroxyacetophenone oxime[J]. Analyst, 1989,114(4):493. doi: 10.1039/an9891400493
|
| [29] |
Al Rawahi W A, Ward N I. Field-based application of developed solid phase extraction with inductively coupled plasma mass spectrometry for vanadium speciation analysis of groundwaters from Argentina[J]. Talanta, 2017,165:391−397. doi: 10.1016/j.talanta.2016.12.078
|
| [30] |
Jen J F, Wu M H, Yang T C. Simultaneous determination of vanadium(IV) and vanadium(V) as EDTA complexes by capillary zone electrophoresis - ScienceDirect[J]. Analytica Chimica Acta, 1997,339(3):251−257. doi: 10.1016/S0003-2670(96)00482-5
|




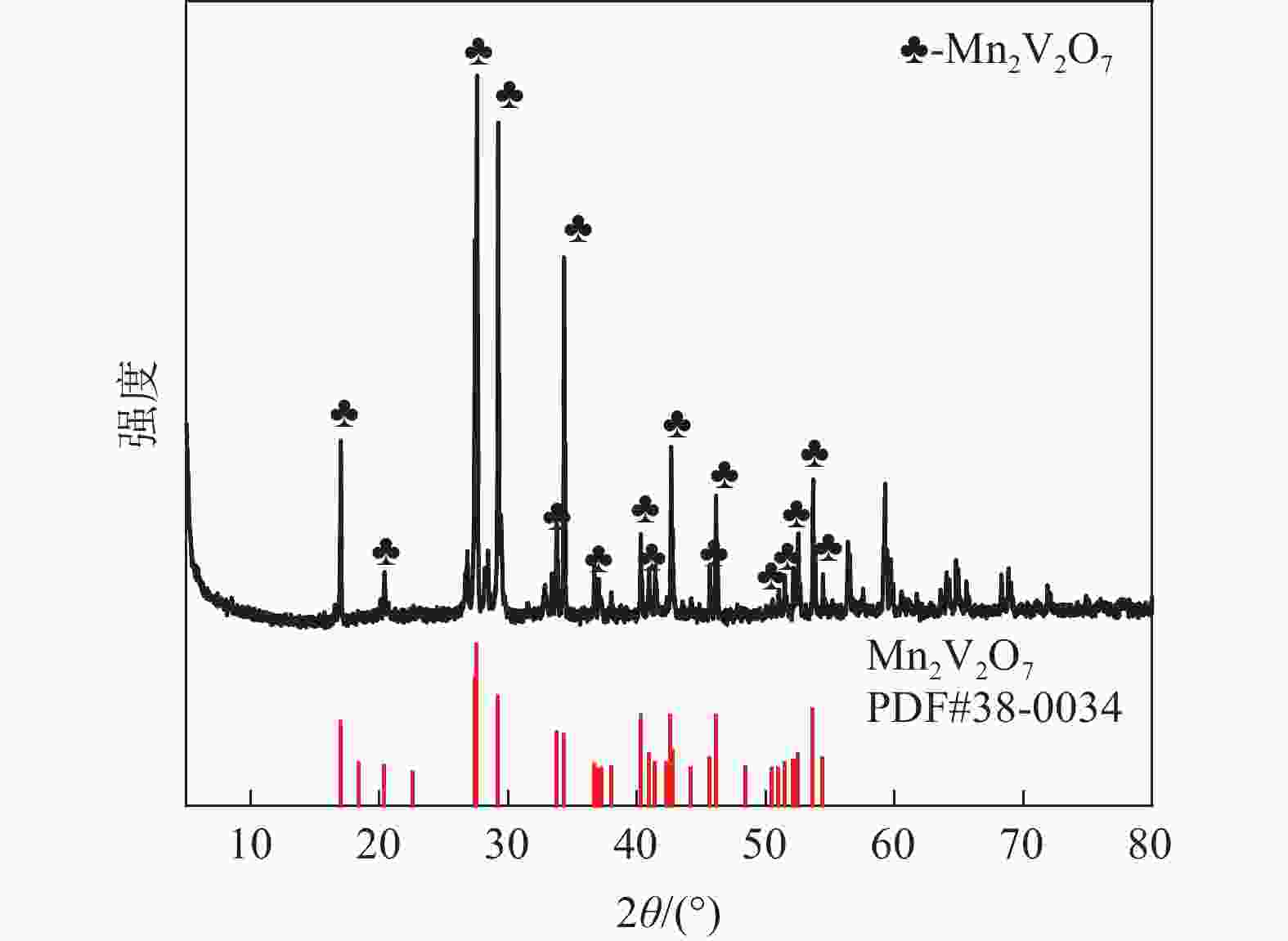
 下载:
下载: