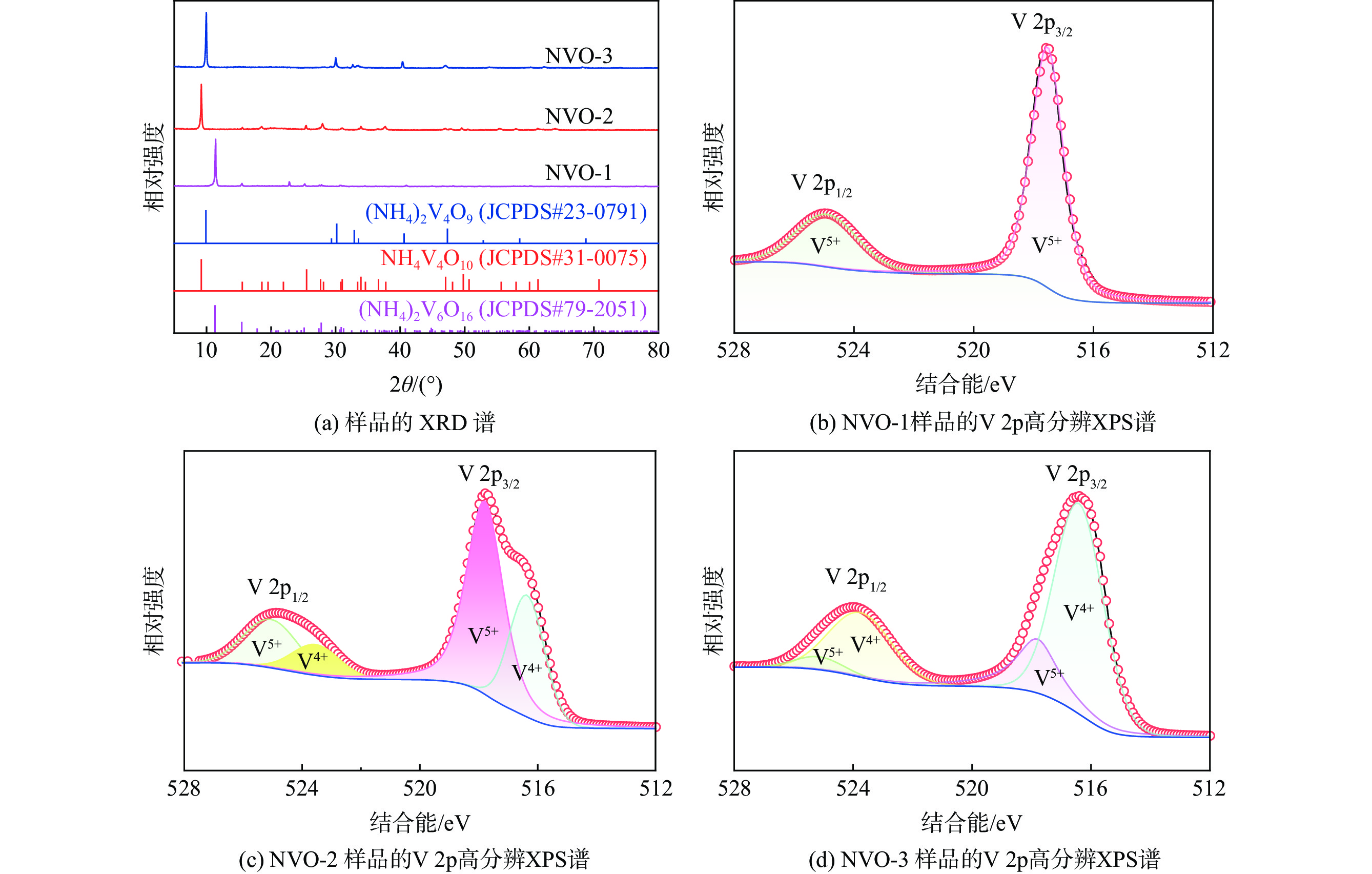
| [1] |
Randau Simon, Weber Dominik A, Kötz Olaf, et al. Benchmarking the performance of all-solid-state lithium batteries[J]. Nature Energy, 2020,5(3):259−270. doi: 10.1038/s41560-020-0565-1
|
| [2] |
Ma Zhuo, Wang Kaixuan, Qiu Yunfeng, et al. Nitrogen and sulfur co-doped porous carbon derived from bio-waste as a promising electrocatalyst for zinc-air battery[J]. Energy, 2018,143:43−55. doi: 10.1016/j.energy.2017.10.110
|
| [3] |
Ma T, Wu S, Wang F, et al. Degradation mechanism study and safety hazard analysis of overdischarge on commercialized lithium-ion batteries[J]. ACS Appl Mater Interfaces, 2020,12(50):56086−56094. doi: 10.1021/acsami.0c18185
|
| [4] |
Essl Christiane, Golubkov Andrey W, Gasser Eva, et al. Comprehensive hazard analysis of failing automotive lithium-ion batteries in overtemperature experiments[J]. Batteries, 2020,6(2):30. doi: 10.3390/batteries6020030
|
| [5] |
Alfaruqi Muhammad H, Mathew Vinod, Song Jinju, et al. Electrochemical zinc intercalation in lithium vanadium oxide: A high-capacity zinc-ion battery cathode[J]. Chemistry of Materials, 2017,29(4):1684−1694. doi: 10.1021/acs.chemmater.6b05092
|
| [6] |
Ming Jun, Guo Jing, Xia Chuan, et al. Zinc-ion batteries: Materials, mechanisms, and applications[J]. Materials Science and Engineering:R:Reports, 2019,135:58−84. doi: 10.1016/j.mser.2018.10.002
|
| [7] |
Cai Kexing, Luo Shaohua, Feng Jie, et al. Recent advances on spinel zinc manganate cathode materials for zinc-ion batteries[J]. Chemical Record, 2021,22(1):1−24.
|
| [8] |
Liu Zhexuan, Qin Liping, Chen Xingyu, et al. Improving stability and reversibility via fluorine doping in aqueous zinc–manganese batteries[J]. Materials Today Energy, 2021,22:100851. doi: 10.1016/j.mtener.2021.100851
|
| [9] |
Heng Yongli, Gu Zhenyi, Guo Jinzhi, et al. Research progresses on vanadium-based cathode materials for aqueous zinc-ion batteries[J]. Acta Physico-Chimica Sinica, 2021,37(3):17−32. (衡永丽, 谷振一, 郭晋芝, 等. 水系锌离子电池用钒基正极材料的研究进展[J]. 物理化学学报, 2021,37(3):17−32.Heng Yongli, Gu Zhenyi, Guo Jinzhi, et al. Research progresses on vanadium-based cathode materials for aqueous zinc-ion batteries[J]. Acta Physico-Chimica Sinica, 2021, 37(3): 17-32
|
| [10] |
Zong Quan, Du Wei, Liu Chaofeng, et al. Enhanced reversible zinc ion intercalation in deficient ammonium vanadate for high-performance aqueous zinc-ion battery[J]. Nano-Micro Letters, 2021,13(1):116. doi: 10.1007/s40820-021-00641-3
|
| [11] |
Bai Youcun, Zhang Heng, Hu Qin, et al. Tuning the kinetics of binder-free ammonium vanadate cathode via defect modulation for ultrastable rechargeable zinc ion batteries[J]. Nano Energy, 2021,90:106596. doi: 10.1016/j.nanoen.2021.106596
|
| [12] |
Prześniak-Welenc Marta, Nadolska Małgorzata, Nowak Andrzej P, et al. Pressure in charge neglected parameter in hydrothermal synthesis turns out to be crucial for electrochemical properties of ammonium vanadates[J]. Electrochimica Acta, 2020,339:135919. doi: 10.1016/j.electacta.2020.135919
|
| [13] |
Sarkar S, Veluri Ps, Mitra Sagar. Morphology controlled synthesis of layered NH4V4O10 and the impact of binder on stable high rate electrochemical performance[J]. Electrochimica Acta, 2014,132:448−456. doi: 10.1016/j.electacta.2014.03.144
|
| [14] |
Kang Wenpei, Zhao Chenhao, Liu Rui, et al. Ethylene glycol-assisted nanocrystallization of LiFePO4 for a rechargeable lithium-ion battery cathode[J]. Cryst Eng Comm, 2012,14(6):2245−2250. doi: 10.1039/c2ce06423e
|
| [15] |
Sheng Rui, Hou Lihua, Wang Lei, et al. Morphology-modulation of (NH4)2V4O9 nanostructures for enhanced electrochemical performance as cathode material for aqueous rechargeable zinc ion batteries[J]. Solid State Ionics, 2022,385:116023. doi: 10.1016/j.ssi.2022.116023
|
| [16] |
Lu Chao, Yang Zhi, Ding Yi, et al. Enhanced electrochemical performance of ammonium vanadate (NH4V4O10) cathode for rechargeable aqueous zinc-ion batteries by altering pH regulators[J]. Materials Today Communications, 2023,35:105993. doi: 10.1016/j.mtcomm.2023.105993
|
| [17] |
Fei Hailong, Wu Xiaomin, Li Huan, et al. Novel sodium intercalated (NH4)2V6O16 platelets: High performance cathode materials for lithium-ion battery[J]. Journal of Colloid and Interface Science, 2014,415:85−88. doi: 10.1016/j.jcis.2013.10.025
|
| [18] |
Sun Rui, Qin Zhaoxia, Liu Xinlong, et al. Intercalation mechanism of the ammonium vanadate (NH4V4O10) 3D decussate superstructure as the cathode for high-performance aqueous zinc-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2021,9(35):11769−11777.
|
| [19] |
Cui Fuhan, Hu Fang, Yu Xin, et al. In-situ tuning the NH4+ extraction in (NH4)2V4O9 nanosheets towards high performance aqueous zinc ion batteries[J]. Journal of Power Sources, 2021,492:229629. doi: 10.1016/j.jpowsour.2021.229629
|
| [20] |
Jeong Unyong, Wang Yuliang, Ibisate Marta, et al. Some new developments in the synthesis, functionalization, and utilization of monodisperse colloidal spheres[J]. Advanced Functional Materials, 2005,15(12):1907−1921. doi: 10.1002/adfm.200500472
|
| [21] |
Liu Hongying, Liang Xiaoping, Jiang Tao, et al. Analysis of structural morphological changes from 3D OM V2O5 film to V2O5 nanorods film and its application in electrochromic device[J]. Solar Energy Materials and Solar Cells, 2022,238:111627. doi: 10.1016/j.solmat.2022.111627
|
| [22] |
Zhang Yifu, Jiang Hanmei, Xu Lei, et al. Ammonium vanadium oxide [(NH4)2V4O9] sheets for high capacity electrodes in aqueous zinc ion batteries[J]. ACS Applied Energy Materials, 2019,2(11):7861−7869. doi: 10.1021/acsaem.9b01299
|
| [23] |
Meng Jiashen, Liu Ziang, Niu Chaojiang, et al. A synergistic effect between layer surface configurations and K ions of potassium vanadate nanowires for enhanced energy storage performance[J]. Journal of Materials Chemistry A, 2016,4(13):4893−4899. doi: 10.1039/C6TA00556J
|
| [24] |
Xu Lei, Zhang Yifu, Jiang Hanmei, et al. Facile hydrothermal synthesis and electrochemical properties of (NH4)2V6O16 nanobelts for aqueous rechargeable zinc ion batteries[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2020,593:124621. doi: 10.1016/j.colsurfa.2020.124621
|
| [25] |
Esparcia Eugene A, Chae Munseok S, Ocon Joey D, et al. Ammonium vanadium bronze (NH4V4O10) as a high-capacity cathode material for nonaqueous magnesium-ion batteries[J]. Chemistry of Materials, 2018,30(11):3690−3696. doi: 10.1021/acs.chemmater.8b00462
|
| [26] |
Lu Chao, Yang Zhi, Wang Yujie, et al. Effect of hydrothermal reaction time on electrochemical properties of (NH4)2V4O9 as cathode material for aqueous zinc ion batteries[J]. Iron Steel Vanadium Titanium, 2022,43(4):62−68. (卢超, 杨智, 汪玉洁, 等. 水热反应时间对水系锌离子电池正极材料(NH4)2V4O9电化学性能的影响[J]. 钢铁钒钛, 2022,43(4):62−68.Lu Chao, Yang Zhi, Wang Yujie, et al. Effect of hydrothermal reaction time on electrochemical properties of (NH4)2V4O9 as cathode material for aqueous zinc ion batteries[J]. Iron Steel Vanadium Titanium, 2022, 43(4): 62-68
|
| [27] |
Zhu Kaiyue, Wu Tao, Huang Kevin. NaCa0.6V6O16· 3H2O as an ultra-stable cathode for Zn-ion batteries: The roles of pre-inserted dual-cations and structural water in V3O8 layer[J]. Advanced Energy Materials, 2019,9(38):1901968. doi: 10.1002/aenm.201901968
|
| [28] |
Lu Chao, Yang Zhi, Wang Yujie, et al. Effect of pH regulation on zinc-storage performance of (NH4)2V4O9 electrode materials[J]. China Nonferrous Metallurgy, 2022,51(5):1−7. (卢超, 杨智, 汪玉洁, 等. pH值调控对(NH4)2V4O9电极材料储锌性能的影响[J]. 中国有色冶金, 2022,51(5):1−7.Lu Chao, Yang Zhi, Wang Yujie, et al. Effect of pH regulation on zinc-storage performance of (NH4)2V4O9 electrode materials[J]. China Nonferrous Metallurgy. 2022, 51(5): 1-7
|
| [29] |
Pan Zikang, Ru Qiang, Zheng Minghui, et al. Construction of hierarchical flower-shaped (NH4) 2V3O8/rGO with enhanced zinc storage performance[J]. Chem Electro Chem, 2021,8(23):4618−4824.
|
| [30] |
Zheng Jiqi, Liu Chaofeng, Tian Meng, et al. Fast and reversible zinc ion intercalation in Al-ion modified hydrated vanadate[J]. Nano Energy, 2020,70:104519. doi: 10.1016/j.nanoen.2020.104519
|




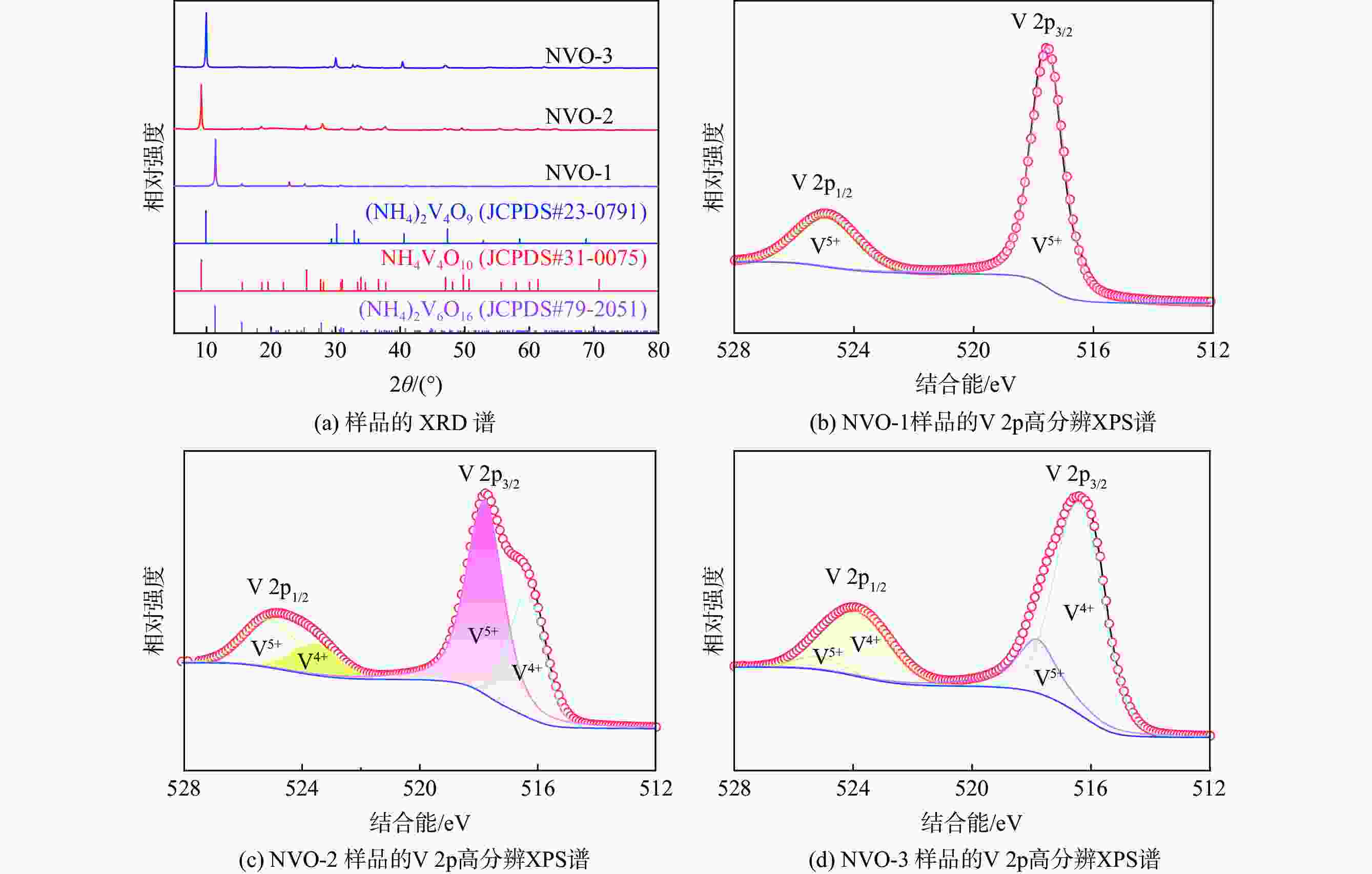
 下载:
下载: